- Experiments

Instant crystals
Make a liquid crystallize with a light touch!
- Sodium acetate
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Take the test tube out of the beaker, open it, and add 1 mL of water. Then continue heating. Now the crystals should melt.
No problem! Just continue heating the test tube for another 5–10 minutes or until everything has melted.
This is normal – the test tube can warp slightly at high temperatures. Keep going; this won’t affect the experiment!
If there are just a few crystals, you can continue the experiment – take the test tube out of the flask and immerse a stick in the liquid. If the test tube is almost completely overgrown with crystals, repeat the experiment from step 4.
The liquid may not have cooled down completely. Wait 10 minutes and try touching the solution with the stick again. If nothing happens, repeat the experiment from the beginning.
Yes; the acetate in the same test tube can be melted and cooled multiple times.
Step-by-step instructions
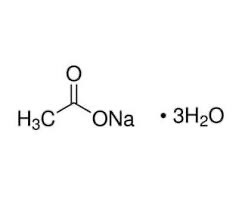
Prepare a stick with sodium acetate CH 3 COONa · 3H 2 O crystals adhered to it. You’ll need it at the very end of the experiment.
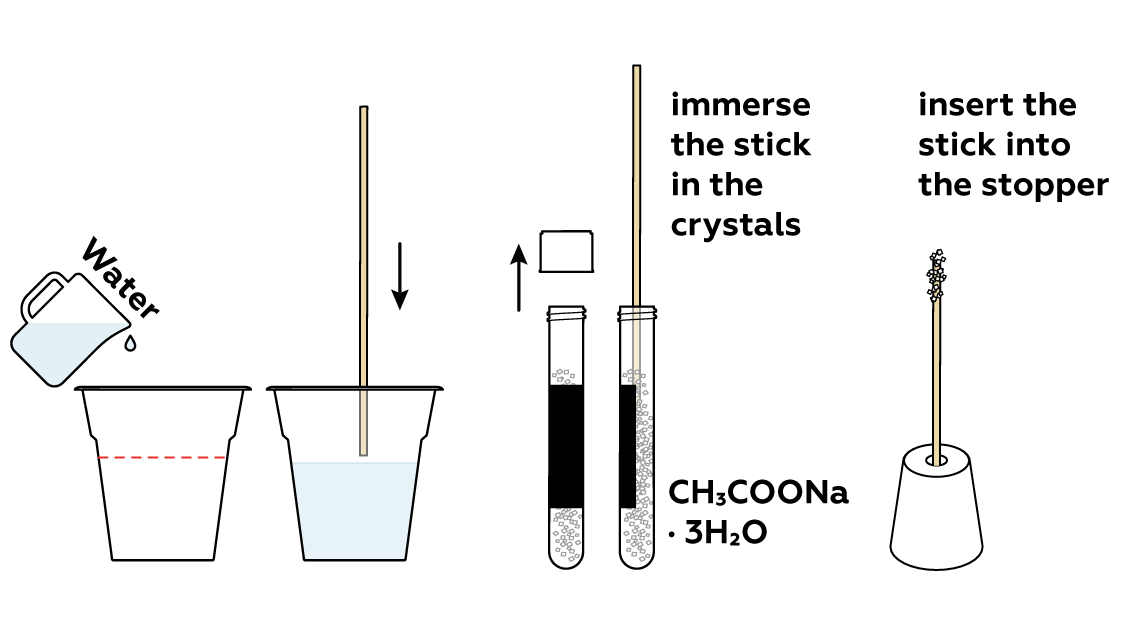
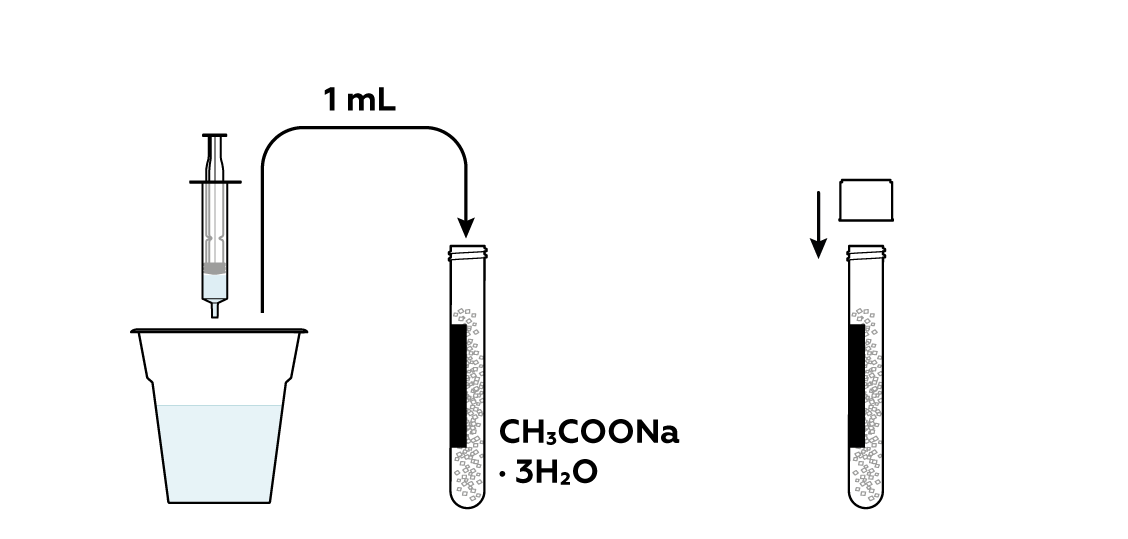
Add some water to the rest of the sodium acetate CH 3 COONa · 3H 2 O crystals. This will help them to melt.
Now, build a heated bath to melt the crystals.
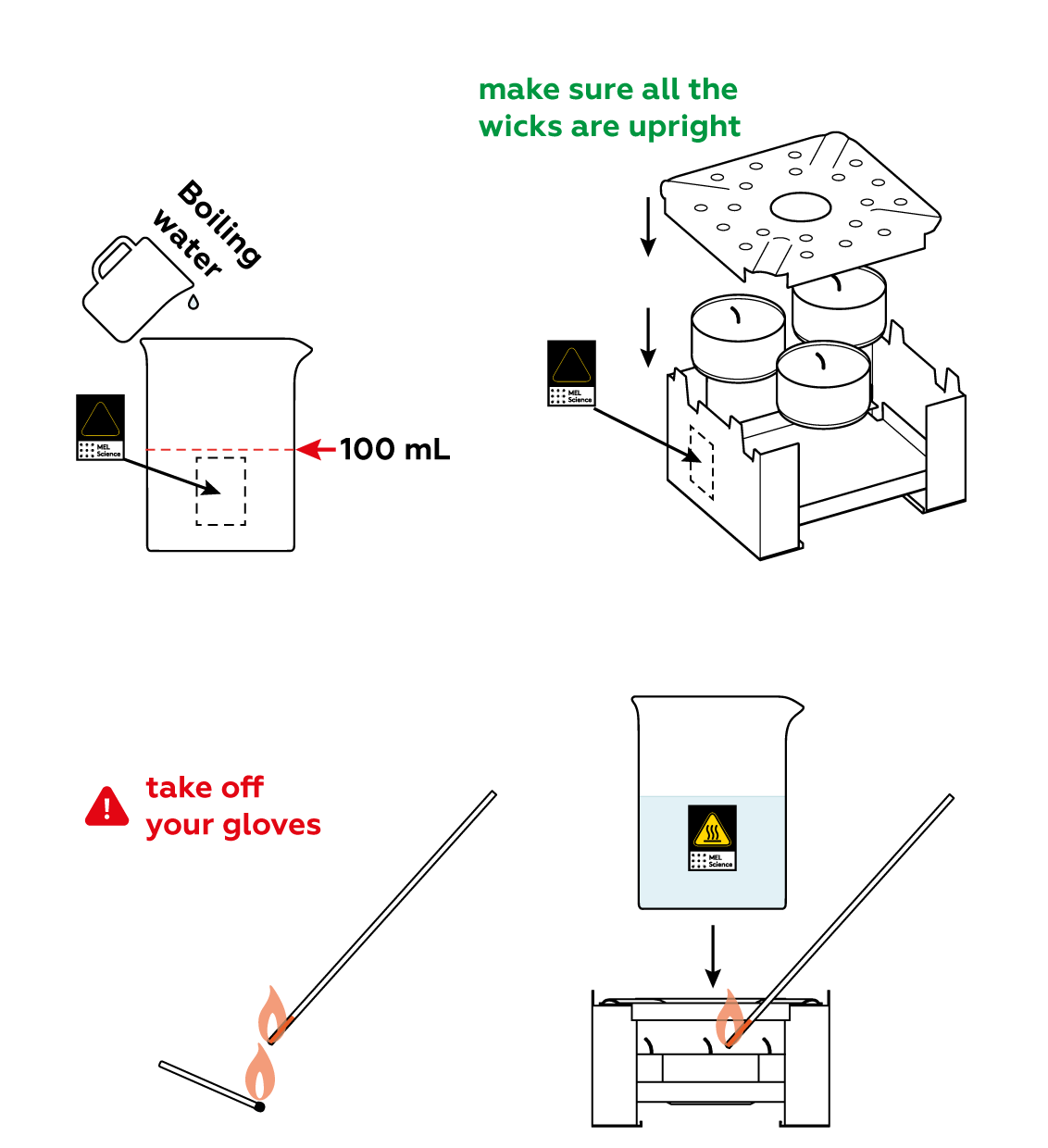
Place the test tube in the glass beaker and watch what happens! The crystals melt as they absorb the heat you’re providing.
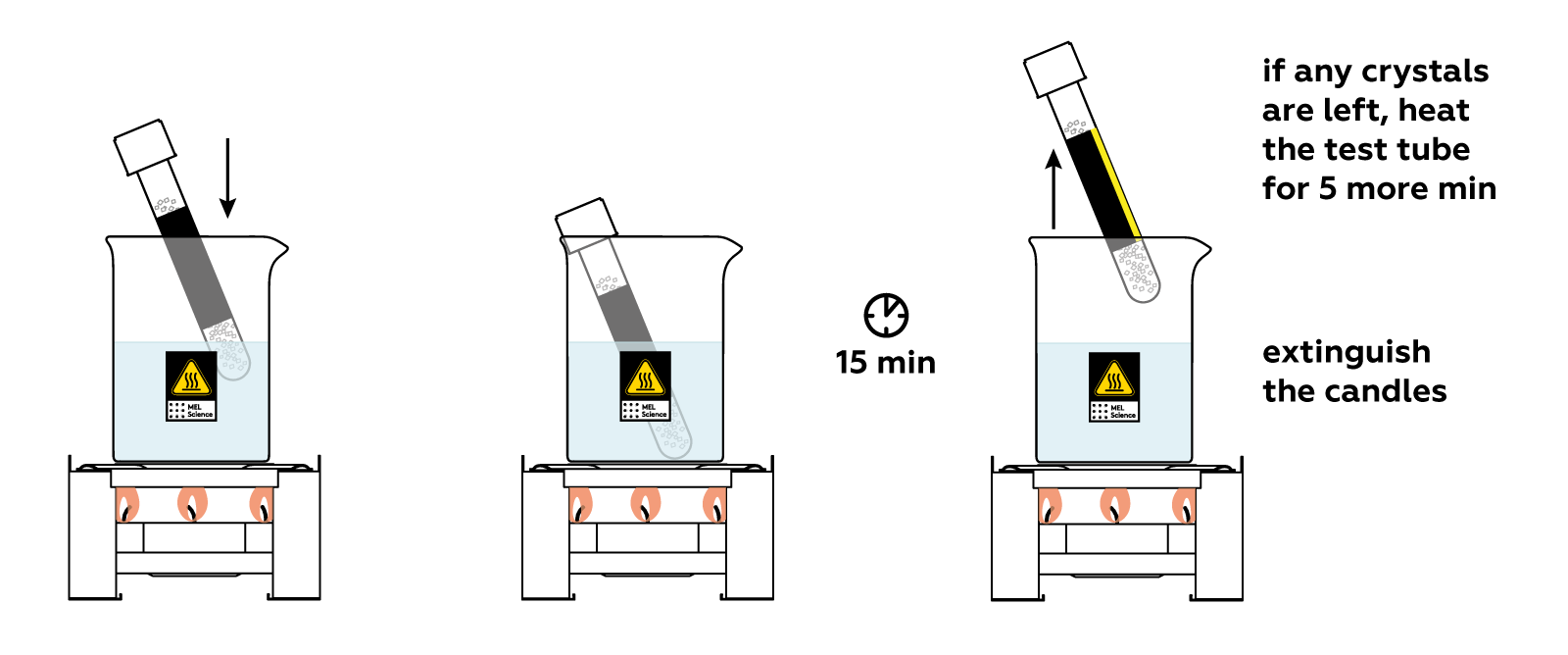
Cool the test tube quickly and carefully. At first, nothing seems to be happening. But just a soft touch of the stick triggers rapid crystal formation! And the test tube heats up dramatically.
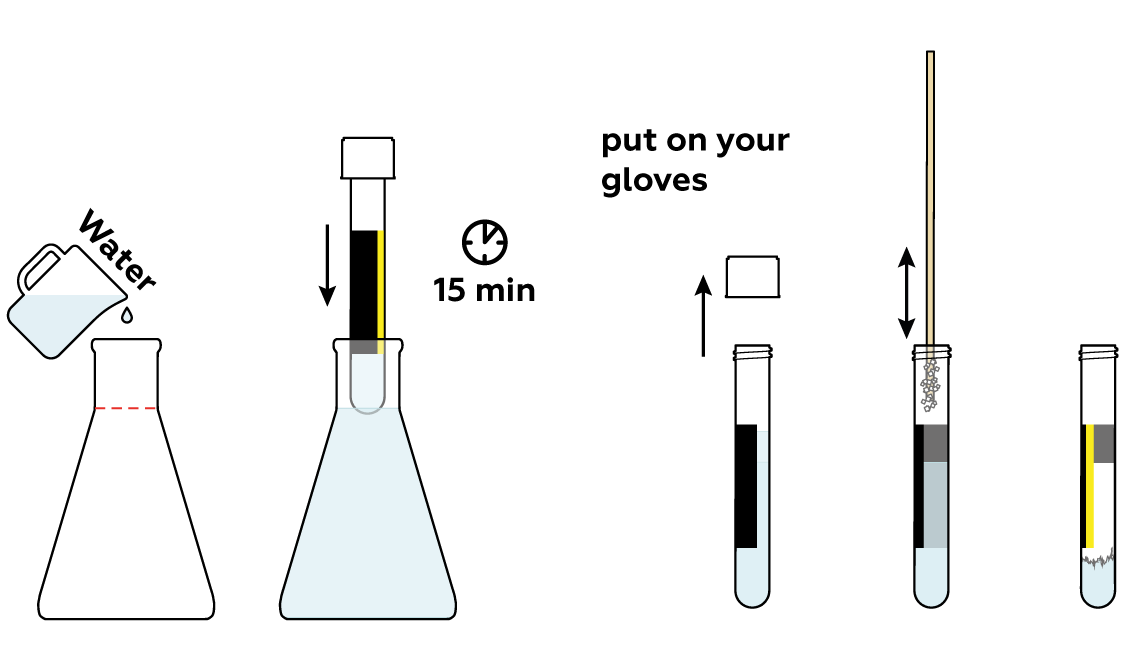
You can conduct this experiment several times! Just repeat steps 3–5.
- Please refer to local regulations when disposing of chemicals.
- Dispose of other solid waste with household garbage.
- Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
When a liquid is cool enough to crystallize but remains a liquid nonetheless, we call it a "supercooled liquid." This state is usually quite unstable, and not only actual crystals, but even a speck of dust can "remind" such a liquid to turn into a crystal.
Can water be supercooled too?
Of course! You can even try to do it at home. Check it out!
For this purpose, you will need to put a half-liter (one pint) bottle of purified water in the freezer for about three hours. When you take it out of the freezer, be gentle – the water is ready to turn into ice! Hit the bottle against the table or shake it and see how quickly it freezes!
Have you already tried it? Watch this video to see what other cool tricks you can do with supercooled water!
If supercooled liquids exist, can superheated ones exist too?
Any substance can exist in one of three states: solid, liquid, or gaseous. For water, these are ice, water, and vapor. As a rule, at normal pressure, the transition from one state to another occurs at a certain temperature: water turns into ice at 0 °C (32 °F), and becomes vapor at 100 °C (212 °F).
However, as you already know, there is an exception to this rule. When gently cooled below their freezing point, the particles that make up a liquid slow down but do not settle into their solid form. But can a liquid be just as gently heated above its boiling point and not start to evaporate? Turns out, it’s possible! And such a liquid is indeed said to be superheated.
In liquids, particles move chaotically and relatively quickly, while still remaining quite close to each other. When heated to their boiling point, they begin to move even faster and detach from the surface of the liquid, turning into a gas. However, if the liquid is heated very carefully, the particles in it will move as fast as in a gas, but will not “dare” to leave the surface. When such a liquid is disturbed, ripples form on its surface, upon which the particles instantly “realize” that it is high time for them to scatter. This phenomenon sometimes occurs when water or milk is heated in a smooth, clean cup in the microwave for a long time. The liquid can seem tranquil at first, but once something like a spoon or a tea bag is immersed in it, the disturbance can trigger the liquid to boil vigorously or even explode! If hot droplets land on the skin, they can cause serious burns, so be careful when heating liquids in the microwave for extended periods of time!
That’s interesting!
Supercooled water in nature.
The supercooling of water underlies the natural phenomenon of freezing rain. If it rains at temperatures below 0 °C (32 °F), the raindrops are supercooled. When they come into contact with any surfaces, such as the ground, tree branches, or power lines, they instantly freeze, forming a thin film of ice. This can cause a plethora of problems: power lines and tree branches can break under the weight of the ice, and ice on the roads can lead to car accidents.

Dozens of experiments you can do at home
One of the most exciting and ambitious home-chemistry educational projects The Royal Society of Chemistry
Super Saturation Experiment: Hot Ice

Introduction: Super Saturation Experiment: Hot Ice

Step 1: Getting the Equipment

Okay Step one is finding or buying any the material that you may need. Below there is suggested equipment to be used. The time of the actual experiment preparation should take in between one to two hours. The presentation is the phase change which can occur in seconds.
Step 2: Getting the Material

Step two decide what material you have or are going to buy. There are two material options below. Select one do not mix these two. This is because they have very different melting temperature and measurements for water become complex.
Step 3: Select How Much Material Needed

Step three is finding the amount of material you will be using to for the experiment. This step is mainly for those who chose to use the material sodium acetate in their experiment. Below are charts and graphs that will help you determine how much water is needed to create sodium acetate trihydrate. Sodium acetate trihydrate is water saturated with sodium acetate. Table of Contents (pictures/charts) 1) Parries hand book data 2) phase diagram of sodium acetate Determine an amount of water to add as long as there is less water per sodium acetate (for people have trihydrate use the trihydrate row) needed to dissolve all the sodium acetate at room temperature (about 20 degrees Celsius). The measurement needed should look something like: (x amount of sodium acetate)/(100g of water) at 70 degrees Celsius. x in this case will be 146g of sodium acetate in this case. for picking the temperature it is highly recomended picking 70 degrees Celsius. When heating the solution we will heat it higher than you measured for so that water is fed in excess. This is so no sodium acetate crystals are left, just one will mess up the experiment. The phase diagram is more for sodium acetate trihydrate showing that its okay to add water to solution, but adding to much will alter the experiment.
Step 4: Review Safety
Step four is reviewing safety. Before even starting an experiment you should read the safety information about the reagents. The safety information may be found online or on the side of the bottle. Sodium acetate and sodium acetate trihydrate have the same safety measures. Sodium Acetate (Safe for class use) NFPA Rating: (estimated) Health: 1; Flammability: 1; Reactivity: 0 Hazards Eye: May cause eye irritation. Skin: May cause skin irritation. Ingestion: Ingestion of large amounts may cause gastrointestinal irritation. Inhalation: No hazard expected in normal industrial use. Chronic: No information found. First aid Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid. Inhalation: Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear. Notes to Physician: None Physical properties Clean up: Is water soluble, use water and dispose of properly. More information at: http://avogadro.chem.iastate.edu/msds/naoac-3h2o.htm
Step 5: Measuring the Material

Step five is where the action of measuring out the material takes place. Using a weigh boat measure your calculated amount of sodium acetate needed in part three*. Measure the sodium acetate in on your chosen scale. If you are using option one then you will need to use water too. Measure out your calculated corresponding amount of water from parries handbook**. Add measured content to a beaker. *If you have sodium trihydrate measuring the amount is not necessarily needed, but is good practice. **If using option two use column two in parries handbook (recommended temperature 30 degrees Celsius or lower), this is optional for option two. Material used: option one, option two . Suggested Equipment: Large Weigh boat, graduated cylinder 100mL, beaker 250mL, electric scale.
Step 6: Heating the Solution

Step six is a mildly difficult step. in this step you will be heating the solution melting any sodium acetate trihydrate. Option one : Here you will place you beaker holding the solution onto a heating device where you may heat the solution to around 70 degrees Celsius * . Stirring with a stir rod is recommended for two reasons while heating the solution. The first reason is to stop sodium acetate trihydrate from burning if heated at hot temperatures. The second reason is to make sure all the solution has melted. A thermometer is also recommended for an accurate read of the temperature of the melted solution. This step should take about 10 to 15 minutes ** . * Sodium acetate trihydrate melts around 58 degrees, however it could take up to around 65 degrees to melt it completely. Also if using sodium acetate you have a second factor: solution must saturate which may require more heat. I would recommend adding an extra 10 degrees Celsius on to what you calculated for to make sure the water is in excess in the solution. ** The difficult part is knowing when the solution has fully melted. What to look for is to see if there are any parts of the liquid that look like ice, or even a needle. Material used : solution in the beaker. Suggested Equipment : beaker 250mL, Stirring rod, thermometer, heating device. Option two : Here we will fill a water bath with enough water to cover most of the beaker. If the beaker starts to float you may hold it down so it will not tip over or place a heave Cleaned object to weigh down the beaker. With a water bath the solution will be a lot less likely to burn and the solution will melt everywhere the water covers. The time it will take to heat the water varies, but can take about ten minutes to get the water the same temperature as the heater. Heat the solution for about twenty minutes. when deciding whether the solution is ready or not place the thermometer in the solution not the water bath. If the temperature is above your calculated/recommended temperature then the solution is ready. Material used: solution in the beaker, tap water . Suggested Equipment: beaker 250mL, Stirring rod, thermometer, heating device, glass bowl.
Step 7: Transferring the Solution to Clean Container

Step seven is an optional, but a strongly recommended step if you just completed option one because the heating device does cover the whole beaker. Here we will transfer the solution to a clean container while the solution is still hot (above 65 degrees Celsius). When pouring the solution make sure if any unmelted crystals that may have stuck to the beakers sides while melting do not fall in the new beaker. It is recommended that you briefly heat the solution again at the same temperature in step six for the the new beaker to make sure that there are no solid crystal formations left. Material used: solution in the beaker. Suggested Equipment: two beakers 250mL, Stirring rod, thermometer, heating device.

Step 8: Cooling the Solution

Step eight is when we take the solution away from the heat source and allowed to cool to room temperature. This step takes about 30 minutes. At this step you may use a refrigerator to speed the cooling process. It is not advised to hover hand or touch the solution with a glove after this point to avoid contamination. Please note this is the step that will show if there was any solid formation of sodium acetate trihydrate left. Check on the solution once after ten minutes to make sure no crystals are forming * . After about 30 minutes the solution is ready for presentation, or may be stored for another day since it is a stable solution. * If there are any crystals forming, that means there was an unmelted crystal that has touched the solution. The solution is still okay, it is a reversible reaction. Go back to step six and reheat the crystallizing solution. if it happens again try heating at a higher temperature. If the problem persists then try using option 2 for heating. Material used: solution in the beaker. Suggested Equipment: two beakers 250mL, refrigerator.
Step 9: Presentation

Step nine is the step that is the step used for display. There are two common options used to display this super saturation phenomenon, however you may choose to be creative and make your own. Few things to know before presenting: 1) When the reaction starts and the sodium acetate trihydrate changes phases from a liquid to a solid, the temperature will heat up to around 58 degrees Celsius. That is about how hot your tap water can get, most house hold have their water set at 52 degrees Celsius. This is not anything dangerous but can be unpleasant if touching while reaction is occurring. 2) The solution will not be set off by any disturbance, like touching the solution. Be cautious though because it is possible to set it off accidentally with any crystals still laying around. 3) The reaction does release energy, but most energy released is done so by temperature change. 4) This reaction has duration measured in seconds and milliseconds depending on the volume so plan accordingly. 5) Option two is not recommend if you had trouble cooling your solution because their may be crystals on the rim of the beaker. 6) If any part of the solution is above 58 degrees Celsius it will not change phases. Option one : Have a seed crystal * on your finger tip, it just needs to be one. Push the seed crystal into the middle of the beaker so the reaction begins in the middle of the beaker. This display is done in seconds, but is great for taking good pictures. *reminder seed crystal is the material sodium acetate trihydrate or sodium acetate that you used in the beginning. option two: H ave a seed crystal on a cleanable surface, it is recommended to use a petri dish because cleanup requires large amounts of water. place the seed crystal in the center of where you will be pouring your solution in the beaker. Begin pouring on the seed crystal slowly to get a base. Once the base has crystallized you may continue pouring making any type of crystal structure. The time of this reaction may be controlled by how fast you pour. The reaction is fast enough to clime a steady stream and start crystallizing the solution in the beaker. This display is great for demonstrations. Material used: solution in the beaker. Suggested Equipment: beakers 250mL, petri dish.
Step 10: Clean Up
Step ten is cleanup after the presentation. Sodium acetate trihydrate is a non hazardous material and you may use may be disposed of the crystals or store them in general storage space. The disposal code for sodium acetate trihydrate is 26a meaning it can be disposed of in a landfill. For cleaning the remaining crystals on the surface the usual options are water, soap water, or acetone. Do not use strong oxidizers around the sodium acetate trihydrate. For sodium acetate trihydrate, which is the end product no mater what you used in the beginning, use tap water is best. Warm water will dilute the sodium acetate making the solution unsaturated. The unsaturated solution is a liquid and may be easily flushed out.
- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information
Lab 18 exp 1 - buffer solution lab
General chemistry ii (chem 1120), motlow state community college, recommended for you, students also viewed.
- Learning p Ka
- Lab station worksheet
- GC 4253 L18 Buffer Solutions
- GC 4253 L23 Exp01 Copper Plating
- GC 4253 L09 Exp01 Specific Heat Metal
- GC 4253 L05 Exp01 Qualitative Analysis 8.39.39 PM
Related documents
- Examination of Physical and Chemical Properties, revised
- Chapter 13 Answers
- GC 4253 L02 Exp01 Acid Base Neutralization
- GC 4253 L09 Exp02 Hand Warmers
Preview text
Experiment 1preparing a, experiment inventory, 25 ml of 4% acetic acid, c 2 h 4 o 2, 5 g of sodium acetate, nac 2 h 3 o 2, *permanent marker, *distilled water, *camera / smart phone, *paper towels, 10 ml graduated cylinder, 100 ml graduated cylinder, (6) 100 ml beakers, metal spatula, glass stir rod.
Note: You must provide the materials listed in *red.
EXPERIMENT 1: PREPARING A BUFFER
In this experiment, you will create a buffer solution using acetic acid and sodium acetate. You will create buffers containing various volumes of these two solutions, measure how these amounts affect overall pH using a pH meter, and examine how these solutions work together to create a buffer.
Put on your safety glasses and gloves (provided in your safety box).
Turn on the scale by pressing the button labeled “0/T.” If your scale does not turn on, you may have to remove the battery cover and remove a small strip of plastic from the battery housing. Once the scale is on, press the “0/T” button a second time to zero the scale. Make sure that the units are in grams (g). If not, press the M button until the units are displayed in grams.
Place a weigh boat on the scale and zero the mass of the weigh boat by pressing the “0/T.” Use your metal spatula to weigh 4 g of sodium acetate. Record the exact mass of the sodium acetate in Table 3.
Transfer the sodium acetate into a 100 mL beaker.
Use the 100 mL graduated cylinder to measure and pour 100 mL of distilled water into the beaker to dissolve the sodium acetate.
Stir the solution with a stir rod until a homogenous solution forms.
Calculate the molarity of this solution and label the beaker with the correct concentration and name of the solution. Record the molarity (moles of solute/liters of solution) in Table 3. (The molar mass of NaC 2 H 3 O 2 is 82 g.)
Use your camera (or smart phone) to take a picture of the results (your labeled beaker). Be sure to correctly label the picture and send it to your instructor along with the answers to the lab questions. ©2018, eScience Labs
Use a permanent marker to label five clean 100 mL beakers as A, B, C, D, and E.
Follow Table 4 to prepare the buffer solutions for Beakers A, B, C, D, and E. Use the 10 mL graduated cylinder to measure
and pour. Clean the graduated cylinder between pours. 11 this is your first lab using the pH meter, read the instructions that came with it on how to calibrate it. If you do not
properly calibrate the meter prior to using it, your results will be inaccurate. If you have used it before, check the calibration using distilled water. It should read a pH close to 7. 12. Remove the cap from the end of the pH meter and rinse the probe with 10 mL of distilled water. Turn the pH meter on by
moving the switch at the top to the right. Place the meter in each of the buffer solutions to obtain the pH. Rinse the pH meter with distilled H 2 O between each buffer solution. 13. Reserve the remaining sodium acetate solution for use in the next experiment, Experiment 2. A minimum of 20 mL is
needed for this.
©2018, eScience Labs
- Multiple Choice
Course : General Chemistry II (CHEM 1120)
University : motlow state community college.

- More from: General Chemistry II CHEM 1120 Motlow State Community College 25 Documents Go to course

Hot Ice Science Experiments With Sodium Acetate

Hot Ice Experiment With Sodium Acetate
How to Make Hot Ice

Hot Ice Science Experiments Tips & Tricks

No comments:
Post a comment.
Thanks for taking the time to leave a comment- I love reading them! :) ~Heather Lynne
Contact Form
- ScHOOL Sign Up
- Scientist Sign Up
- Our Scientists
- Our Research
- Give us Feedback!
- In the News

'Hot Ice' is when we grow a crystal of sodium acetate from a solution, and doing so we release heat!
This ability to release heat during crystal formation, makes it useful for the reusable hand warmers/heating pads.

How does it work?
Sodium acetate trihydrate crystals melt at 58 °C. The melting is the removal of the tree molecules of water (= thihydrate ) incorporated into the crystal, in whitch the salt disolves, creating a supersaturated solution . It is then possible to cool this solution without the formation of crystals to the room temberature or even lower!
The crystal will start to grow from a nucleation centre, as on the photo below. The nucleation center can be started by putting an object (knife, toothpick, cocktail stick, ...) into the solution or, in the case of handwarmer, a click of the metal disk in the sealed within. As the crystal of sodium acetate trihydrate grows radially across the container, picking up the waters from solution, the heat is released ( exothermic reaction ).
Basile demonstrating the 'hot ice' nucleation during a call
This reaction is reverseable , and so we can reheat the crystals, melting waters away and disolving sodium acetate in them.
Try experiment at home:
You will need:
- 300g of sodium acetate crystals (can be purchaced online)
- very clean glass without scratches
- cocktail stick
- Measure 300g of sodium acetate
- Pour 80ml (5 tablespoons of water for the reactive version) of water into a mixing jug and mix in the crystals of sodium acetate.
- Microwave the mixture for 10-20 seconds and stir it with a metal spoon, repeat as necessary until the solution is perfectly clear (everything is dissolved).
- One everything is dissolved, pour the solution carefully into a very clean glass. P ro Tip 1: first rinse the glass with warm water inside, it will keep it warm and clean. Pro Tip 2: keep the pan as crystals will form at the bottom and can be used to seed the crystallisation process.
- Place the glass with sodium acetate solution into the fridge for at least 2 hours (until the solution is cold).
- Take the glass from the fridge and use a cocktail stick to trigger the crystallisation (if it doesnt work you may need to put a little crystal from the pan onto the cocktail stick and submerge it).
- You can reuse the formed crystal! Just pop it into a microwave to heat up, stir it a little bit and place into a new glass (rinced by warm water)!
- Share your videos and pictures with us via Twitter @Scientist_ND and Facebook @scientistND
- [email protected]

IMAGES
VIDEO