Human autoimmune diseases: a comprehensive update
Affiliations.
- 1 Research Center for Biological Therapy, The Institute of Translational Hepatology, Beijing 302 Hospital, Beijing, China.
- 2 Division of Rheumatology, Allergy and Clinical Immunology, University of California at Davis School of Medicine, Davis, CA, USA.
- PMID: 26212387
- DOI: 10.1111/joim.12395
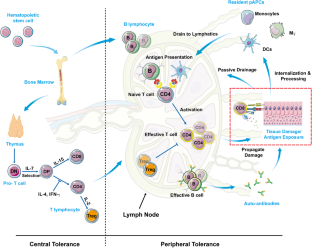
There have been significant advances in our understanding of human autoimmunity that have led to improvements in classification and diagnosis and, most importantly, research advances in new therapies. The importance of autoimmunity and the mechanisms that lead to clinical disease were first recognized about 50 years ago following the pioneering studies of Macfarlane Burnett and his Nobel Prize-winning hypothesis of the 'forbidden clone'. Such pioneering efforts led to a better understanding not only of autoimmunity, but also of lymphoid cell development, thymic education, apoptosis and deletion of autoreactive cells. Contemporary theories suggest that the development of an autoimmune disease requires a genetic predisposition and environmental factors that trigger the immune pathways that lead, ultimately, to tissue destruction. Despite extensive research, there are no genetic tools that can be used clinically to predict the risk of autoimmune disease. Indeed, the concordance of autoimmune disease in identical twins is 12-67%, highlighting not only a role for environmental factors, but also the potential importance of stochastic or epigenetic phenomena. On the other hand, the identification of cytokines and chemokines, and their cognate receptors, has led to novel therapies that block pathological inflammatory responses within the target organ and have greatly improved the therapeutic effect in patients with autoimmune disease, particularly rheumatoid arthritis. Further advances involving the use of multiplex platforms for diagnosis and identification of new therapeutic agents should lead to major breakthroughs within the next decade.
Keywords: Immune tolerance; Immunopathology; autoantibodies; genetics and autoimmunity.
© 2015 The Association for the Publication of the Journal of Internal Medicine.

Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Autoimmune Diseases* / epidemiology
- Autoimmune Diseases* / etiology
- Autoimmune Diseases* / genetics
- Autoimmune Diseases* / therapy
- Environmental Exposure
- Genetic Predisposition to Disease
- Immune Tolerance
- Models, Biological
- Twin Studies as Topic
Grants and funding
- DK39588/DK/NIDDK NIH HHS/United States
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Editorial: Insights in autoimmune and autoinflammatory disorders: 2021
Raphaela goldbach-mansky, betty diamond.
- Author information
- Article notes
- Copyright and License information
Edited and Reviewed by: Luca Quartuccio, University of Udine, Italy
*Correspondence: Raphaela Goldbach-Mansky, [email protected]
This article was submitted to Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders: a section of the journal Frontiers in Immunology
Received 2022 Nov 8; Accepted 2022 Nov 29; Collection date 2022.
Keywords: autoimmunity, autoinflammation, SLE, RA, risk factors, viral pathogenesis, biomarkers
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
The Research Topic: Insights in Autoimmune and Autoinflammatory Disorders: 2021 was implemented to highlight latest advancements in research across the field of Immunology in Autoimmunity and Autoinflammation by discussing recent advances, current challenges, and future perspectives. Under the Research Topic 13 articles were published, 10 contributions highlight topics relevant to autoimmune diseases and 3 to autoinflammation.
In two original research papers two groups set out to identify microbial or viral risk factors that predispose to the development of autoimmunity.
Yin et al. mined microarray datasets from disease-specific target tissues including the pancreas, thyroid, and intestine from individuals with Type 1 Diabetes (T1D), Hashimoto thyroiditis (HT), and celiac disease (CD), as well as matched controls. They discovered viral signatures of common viral infections including influenza A, human T-lymphotropic virus type 1, and herpes simplex that were shared in target tissues of the three autoimmune diseases studied thus pointing to common environmental factors as drivers of autoimmune diseases. Lin et al. assessed a potential role of gut microbiota in driving autoimmune diseases in a retrospective population-based cohort study analyzing data from a Taiwanese Insurance Research Database of over 290,000 patients treated with PPIs in the period between 2002 and 2015. The role of PPIs in modifying gut microbiota has previously been described ( 1 ). The authors show that compared to the non-PPI group, the adjusted hazard ratios (aHR) were higher for several organ specific autoimmune diseases including Grave’s disease, Hashimoto thyroiditis, autoimmune hemolytic anemia, immune thrombocytopenic purpura, Henoch-Schoenlein purpura and Myasthenia gravis and also for systemic autoimmune diseases including ankylosing spondylitis, rheumatoid arthritis, primary Sjogren syndrome, systemic lupus erythematosus, systemic vasculitis, psoriasis, systemic scleroderma and inflammatory myopathies.
Two papers, an original research paper by Yang et al. and a mini review by Chen et al. assess the role of transfer RNA (tRNA)-derived small noncoding RNA (tsRNA), an emerging class of small non-coding RNAs, and the role of ferroptosis in patients with systemic lupus erythematosus (SLE) respectively. Yang et al. assessed the diagnostic value of a potential biomarker, tRF-His-GTG-1, a candidate tsRNA that best differentiated between the SLE and control groups. The tsRNA was first assessed in a training set of 57 SLE patients with and without lupus nephritis and then in a validation set of 52 SLE patients without Lupus nephritis, 83 SLE patients with lupus nephritis and 86 healthy controls; tRF-His-GTG-1 was significantly elevated in serum exosomes from SLE patients compared to healthy controls and its elevation in serum distinguished SLE with lupus nephritis from SLE without lupus nephritis with and AUC of 0.81 (95% CI 0.73-0.88) with high specificity, however, the sensitivity was lower, 66.27%. Pathway analysis predicted that the tsRNA can target signaling pathways including MAPK signaling and RIG-I signaling and EBV infection. Chen et al. review data on the role of ferroptosis, a novel non-apoptotic regulated form of cell death, in SLE.
Two reviews assess mechanisms that lead to fibroblast proliferation and inflammation in rheumatoid arthritis (RA). Jiang et al. review the pathogenic role of secreted frizzled-related protein 1 (SFRP1), a member of the secretory glycoprotein SFRP family, that are thought to “antagonize” the Wnt signaling pathway by interfering es with Wnt signaling transduction. Their role in determining cell fate by regulating cell proliferation, differentiation, apoptosis, and pyroptosis has been previously reported ( 2 ) SFRP1 is widely expressed in human cells, including fibroblast-like synoviocytes (FLS) of rheumatoid arthritis (RA) and in osteoarthritis ( 3 ). The authors summarized data on mechanisms of SFRP1 regulation of RA-FLS pyroptosis through Wnt/β-catenin and Notch signaling pathways and summarize data on the epigenetic regulation of SFRP1 in RA-FLS. They conclude proposing that Wnt/β-catenin and Notch signaling pathways may collaborate in NLRP3-mediated cell pyroptosis and suggest a role of SFRP1 in hypermethylation of synovial tissue from knee joints in patients with RA and OA. The authors further suggest a potential role of inhibition of hypermethylation in the treatment of RA. Zhao et al. assess the role of G-Protein-Coupled Receptors (GPCR) that includes chemokine receptors, melanocortin receptors, lipid metabolism-related receptors, adenosine receptors, and other inflammation-related receptors, on the pathogenesis of RA, in regulating inflammation, lipid metabolism, angiogenesis, and bone destruction. This review provides comprehensive tables on GPCRs and their expression in immune cells synovium and synovial fibroblasts and discusses possible factors that elucidate the failure of clinical trials blocking cytokines in RA. The authors suggest that the widespread expression of chemokine receptors in a variety of cells may imply that a portion of chemokine receptors may be necessary for homeostatic processes, and further point to the fact that the expression of GPCRs at different disease stages of RA may have diverse functional roles.
Three papers address various mechanisms that can modify tissue specific autoimmune effects, including the role of double negative T regulatory cells in regulating tolerance in the female reproductive environment, the role of NETs in IgA vasculitis and an original paper probing the relationship of C3 levels with disease outcomes in patients with glomerular basement membrane (GBM) disease. Bafor et al. review the emerging role of double negative T regulatory cells (DNTregs), (TCRαβ+/γδ+CD3+CD4–CD8–) on regulating immune tolerance in and female reproductive function. The breakdown of immune tolerance leads to ovulation dysfunction, implantation failure, and pregnancy loss. The authors also discuss mechanisms by which DNTregs provide immune tolerance and maintain and restore the balance in the reproductive microenvironment of female fertility. Chen et al. review the pathomechanisms of IgA activated neutrophils and their release of NETs into tissues and the peripheral blood. The authors review the role of NETs in Immunoglobulin A vasculitis (IgAV) in children. They summarize data suggesting that IgA can induce neutrophils to release NETs via Fcα receptor I (FcaRI) and that FcaRI is elevated in children with active IgAV. The authors further suggest that NETs may serve as potential biomarkers to assess disease activity in IgAV. Zhu et al. conducted a retrospective study of 94 anti- glomerular basement membrane (GBM) disease who were seen in the National Clinical Research Center of Kidney Diseases, Jinling Hospital (China) and found that kidney outcomes of anti-GBM disease in the low C3 group were poorer than those in the normal C3 group. Jiang et al. report a patient presenting with coexisting of autoimmune polyglandular syndrome (APS) Type 3 and Gonadotropin-Releasing Hormone Deficiency who presented with secondary amenorrhea.
And finally, 3 original research papers focus on the pathogenesis of three autoinflammatory diseases. The first paper reports a patient with a novel de novo JAK1 mutation causing a keratinization disorder presenting with hepatitis and autism that was examined in a murine model, a second paper reports of a screening method for candidate drugs for Behcet’s disease, candidates were evaluated in a murine experimental autoimmune uveitis (EAU) model, and the third paper describes a novel disease-causing NLRP3 variant that presents as autosomal dominant hearing loss.
Takeichi et al. report a patient with a novel, de novo JAK1 mutation, p.H596D, who presents with generalized papular skin rashes consistent with a keratinization disorder and with early-onset liver dysfunction and autism. Using CRISPR-Cas9 gene targeting, the authors generated mice with the identical JAK1 mutation and describe hyperactivation of tyrosine kinases and NF-κB signaling pathways. To investigate the role of the mutation in neurodevelopment, the authors observed a strong correlation between genes downregulated in the Jak1 H595D/+;I596I/+;Y597Y/+ mice and those downregulated in the brain of model mice with 22q11.2 deletion syndrome that showed cognitive and behavioral deficits, and are used to model autism spectrum disorders. Xia et al. used an in silico approach to identify potential drugs for the treatment of Behcet’s disease that they validated in a mouse model of experimental autoimmune uveitis (EAU) (B10.RIII mice developed EAU after subcutaneous injection of 200 µl of emulsifier per mouse). The biological functions and pathways of the target genes were analyzed in detail by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses and gene drug interactions were identified from the Drug Gene Interaction Database (DGIdb). Of drugs identified to interact with the 3 top hub genes in Behcet’s, rabenprozole and celastrol reduced anterior chamber inflammation in retinal inflammation in EAU mice. Lastly, Oziebloet al. screened 110 autosomal dominant hearing loss (HL) families with a custom panel of 237 HL genes and identified one family carrying a novel NLRP3 mutation, p.S624R that led to HL in 9 patients in a pedigree of 4 generations. Functional studies identified the novel variant as gain-of-function mutation, leading to increased activity of caspase-1 and subsequent oversecretion of the proinflammatory interleukin-1β. Similar to patients with previously reported mutations causing DFNA34, the identified patients did not present with features of systemic inflammation and did not meet the diagnostic criteria for cryopyrin associated autoinflammatory diseases (CAPS) including MWS, NOMID, or FCAS.
Author contributions
Both authors contributed to this editorial RM drafted the editorial and BD approved the final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- 1. Spiegel BM, Chey WD, Chang L. Bacterial overgrowth and irritable bowel syndrome: Unifying hypothesis or a spurious consequence of proton pump inhibitors? Am J Gastroenterol (2008) 103(12):2972–6. doi: 10.1111/j.1572-0241.2008.01992.x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Rodriguez J, Esteve P, Weinl C, Ruiz JM, Fermin Y, Trousse F, et al. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci (2005) 8(10):1301–9. doi: 10.1038/nn1547 [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Ijiri K, Nagayoshi R, Matsushita N, Tsuruga H, Taniguchi N, Gushi A, et al. Differential expression patterns of secreted frizzled related protein genes in synovial cells from patients with arthritis. J Rheumatol (2002) 29(11):2266–70. [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (283.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 14 December 2023
Autoimmune diseases: targets, biology, and drug discovery
- Shu-jie Li 1 , 2 ,
- Yan-li Wu 1 ,
- Juan-hua Chen 1 ,
- Shi-yi Shen 1 , 3 ,
- Jia Duan 1 , 3 , 4 &
- H. Eric Xu 1 , 5
Acta Pharmacologica Sinica volume 45 , pages 674–685 ( 2024 ) Cite this article
2428 Accesses
6 Citations
1 Altmetric
Metrics details
Autoimmune diseases (AIDs) arise from a breakdown in immunological self-tolerance, wherein the adaptive immune system mistakenly attacks healthy cells, tissues and organs. AIDs impose excessive treatment costs and currently rely on non-specific and universal immunosuppression, which only offer symptomatic relief without addressing the underlying causes. AIDs are driven by autoantigens, targeting the autoantigens holds great promise in transforming the treatment of these diseases. To achieve this goal, a comprehensive understanding of the pathogenic mechanisms underlying different AIDs and the identification of specific autoantigens are critical. In this review, we categorize AIDs based on their underlying causes and compile information on autoantigens implicated in each disease, providing a roadmap for the development of novel immunotherapy regimens. We will focus on type 1 diabetes (T1D), which is an autoimmune disease characterized by irreversible destruction of insulin-producing β cells in the Langerhans islets of the pancreas. We will discuss insulin as possible autoantigen of T1D and its role in T1D pathogenesis. Finally, we will review current treatments of TID and propose a potentially effective immunotherapy targeting autoantigens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders

The immunology of type 1 diabetes
Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?)
Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401:1878–90.
Article PubMed Google Scholar
Hampton HR, Chtanova T. Lymphatic migration of immune cells. Front Immunol. 2019;10:1168.
Article CAS PubMed PubMed Central Google Scholar
Griffin JD, Song JY, Sestak JO, DeKosky BJ, Berkland CJ. Linking autoantigen properties to mechanisms of immunity. Adv Drug Deliv Rev. 2020;165:105–16.
Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Ther. 2023;8:235.
Burbelo PD, Iadarola MJ, Keller JM, Warner BM. Autoantibodies targeting intracellular and extracellular proteins in autoimmunity. Front Immunol. 2021;12:548469.
Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10:741–60.
Mahase E. Type 1 diabetes: Global prevalence is set to double by 2040, study estimates. BMJ. 2022;378:o2289.
Katsarou A, Gudbjornsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Prim. 2017;3:1–17.
Google Scholar
Syed FZ. Type 1 Diabetes Mellitus. Ann Intern Med. 2022;175:ITC33–ITC48.
Daneman D. Type 1 diabetes. Lancet (Lond, Engl). 2006;367:847–58.
Article CAS Google Scholar
Norris JM, Johnson RK, Stene LC. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020;8:226–38.
Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15:635–50.
Article CAS PubMed Google Scholar
Geravandi S, Liu H, Maedler K. Enteroviruses and T1D: is it the virus, the genes or both which cause T1D. Microorganisms. 2020;8:1017.
DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–62.
Article PubMed PubMed Central Google Scholar
Karges B, Prinz N, Placzek K, Datz N, Papsch M, Strier U, et al. A comparison of familial and sporadic type 1 diabetes among young patients. Diabetes Care. 2021;44:1116–24.
Sims EK, Besser REJ, Dayan C, Geno Rasmussen C, Greenbaum C, Griffin KJ, et al. Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes. 2022;71:610–23.
Dayan CM, Besser RE, Oram RA, Hagopian W, Vatish M, Bendor-Samuel O, et al. Preventing type 1 diabetes in childhood. Science. 2021;373:506–10.
Article ADS CAS PubMed Google Scholar
Nekoua MP, Alidjinou EK, Hober D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2022;18:503–16.
Sano H, Imagawa A. Re-enlightenment of fulminant type 1 diabetes under the COVID-19 pandemic. Biology (Basel). 2022;11:1662.
CAS PubMed Google Scholar
Del Chierico F, Rapini N, Deodati A, Matteoli MC, Cianfarani S, Putignani L. Pathophysiology of type 1 diabetes and gut microbiota role. Int J Mol Sci. 2022;23:14650.
Krischer JP, Liu X, Vehik K, Akolkar B, Hagopian WA, Rewers MJ, et al. Predicting islet cell autoimmunity and type 1 diabetes: an 8-year TEDDY study progress report. Diabetes Care. 2019;42:1051–60.
Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589–94.
Article ADS CAS PubMed PubMed Central Google Scholar
Warshauer JT, Bluestone JA, Anderson MS. New frontiers in the treatment of type 1 diabetes. Cell Metab. 2020;31:46–61.
Rodriguez-Calvo T, Richardson SJ, Pugliese A. Pancreas pathology during the natural history of type 1 diabetes. Curr Diab Rep. 2018;18:1–12.
Article Google Scholar
Boughton CK, Munro N, Whyte M. Targeting beta-cell preservation in the management of type 2 diabetes. Br J Diabetes. 2017;4:134–44.
Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest. 2017;127:2881–91.
Foulis A, Farquharson M, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:333–43.
Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60.
Bender C, Rajendran S, Von Herrath MG. New insights into the role of autoreactive CD8 T cells and cytokines in human type 1 diabetes. Front Endocrinol. 2021;11:606434.
Nagy N, de la Zerda A, Kaber G, Johnson PY, Hu KH, Kratochvil MJ, et al. Hyaluronan content governs tissue stiffness in pancreatic islet inflammation. J Biol Chem. 2018;293:567–78.
Bonifacio E, Achenbach P. Birth and coming of age of islet autoantibodies. Clin Exp Immunol. 2019;198:294–305.
Achenbach P, Bonifacio E, Koczwara K, Ziegler AG. Natural history of type 1 diabetes. Diabetes. 2005;54:S25–S31.
Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9.
Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark Å, Hagopian WA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–7.
Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–9.
Jacobsen LM, Haller MJ, Schatz DA. Understanding pre-type 1 diabetes: the key to prevention. Front Endocrinol. 2018;9:70.
Culina S, Brezar V, Mallone R. Insulin and type 1 diabetes: immune connections. Eur J Endocrinol. 2013;168:R19–R31.
Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–5.
Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–3.
Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–31.
Baker RL, Delong T, Barbour G, Bradley B, Nakayama M, Haskins K. Cutting edge: CD4 T cells reactive to an islet amyloid polypeptide peptide accumulate in the pancreas and contribute to disease pathogenesis in nonobese diabetic mice. J Immunol. 2013;191:3990–4.
Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–31.
Roep BO, Solvason N, Gottlieb PA, Abreu JR, Harrison LC, Eisenbarth GS, et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8 + T cells in type 1 diabetes. Sci Transl Med. 2013;5:191ra82.
Han B, Serra P, Amrani A, Yamanouchi J, Marée AF, Edelstein-Keshet L, et al. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–52.
Wenzlau J, Walter M, Gardner T, Frisch L, Yu L, Eisenbarth G, et al. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J Clin Endocrinol Metab. 2010;95:4712–9.
Prasad S, Kohm AP, McMahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9-23 epitope and involves functional epitope spreading. J Autoimmun. 2012;39:347–53.
Takeyama N, Ano Y, Wu G, Kubota N, Saeki K, Sakudo A, et al. Localization of insulinoma associated protein 2, IA-2 in mouse neuroendocrine tissues using two novel monoclonal antibodies. Life Sci. 2009;84:678–87.
Kracht MJL, van Lummel M, Nikolic T, Joosten AM, Laban S, van der Slik AR, et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat Med. 2017;23:501–7.
Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–4.
Sperling MA, Laffel LM. Current management of glycemia in children with type 1 diabetes mellitus. N Engl J Med. 2022;386:1155–64.
Gregory JM, Cherrington AD, Moore DJ. The peripheral peril: injected insulin induces insulin insensitivity in type 1 diabetes. Diabetes. 2020;69:837–47.
Gruessner RWG. The current state of clinical islet transplantation. Lancet Diabetes Endocrinol. 2022;10:476–8.
Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–32.
Sims EK, Bundy BN, Stier K, Serti E, Lim N, Long SA, et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med. 2021;13:eabc8980.
Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37:1069–75.
Edner NM, Heuts F, Thomas N, Wang CJ, Petersone L, Kenefeck R, et al. Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes. Nat Immunol. 2020;21:1244–55.
Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2016;125:3285–96.
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–52.
Cabrera SM, Wang X, Chen YG, Jia S, Kaldunski ML, Greenbaum CJ, et al. Interleukin‐1 antagonism moderates the inflammatory state associated with type 1 diabetes during clinical trials conducted at disease onset. Eur J Immunol. 2016;46:1030–46.
Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, et al. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. 2020;383:2007–17.
Rosenzwajg M, Salet R, Lorenzon R, Tchitchek N, Roux A, Bernard C, et al. Low-dose IL-2 in children with recently diagnosed type 1 diabetes: a phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia. 2020;63:1808–21.
Smith EL, Peakman M. Peptide immunotherapy for type 1 diabetes—clinical advances. Front Immunol. 2018;9:392.
Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189.
Honaker Y, Hubbard N, Xiang Y, Fisher L, Hagin D, Sommer K, et al. Gene editing to induce FOXP3 expression in human CD4 + T cells leads to a stable regulatory phenotype and function. Sci Transl Med. 2020;12:eaay6422.
Michalak SS, Olewicz-Gawlik A, Rupa-Matysek J, Wolny-Rokicka E, Nowakowska E, Gil L. Autoimmune hemolytic anemia: current knowledge and perspectives. Immun Ageing. 2020;17:38.
Buerck JP, Burke DK, Schmidtke DW, Snyder TA, Papavassiliou D, O’Rear EA. A flow induced autoimmune response and accelerated senescence of red blood cells in cardiovascular devices. Sci Rep. 2019;9:19443.
Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 2015;350:680–4.
Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98:24–33.
Li J, Sullivan JA, Ni H. Pathophysiology of immune thrombocytopenia. Curr Opin Hematol. 2018;25:373–81.
Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–54.
Duan J, Xu P, Luan X, Ji Y, He X, Song N, et al. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022;609:854–9.
Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Prim. 2019;5:30.
Noridomi K, Watanabe G, Hansen MN, Han GW, Chen L. Structural insights into the molecular mechanisms of myasthenia gravis and their therapeutic implications. Elife. 2017;6:e23043.
Takahashi H, Iriki H, Asahina Y. T cell autoimmunity and immune regulation to desmoglein 3, a pemphigus autoantigen. J Dermatol. 2023;50:112–23.
Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–32.
Miyazaki K, Abe Y, Iwanari H, Suzuki Y, Kikuchi T, Ito T, et al. Establishment of monoclonal antibodies against the extracellular domain that block binding of NMO-IgG to AQP4. J Neuroimmunol. 2013;260:107–16.
Mader S, Brimberg L. Aquaporin-4 water channel in the brain and its implication for health and disease. Cells. 2019;8:90.
Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112:11–8.
Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–63.
Mikasova L, De Rossi P, Bouchet D, Georges F, Rogemond V, Didelot A, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–21.
Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97:839–87.
Gibson LL, McKeever A, Coutinho E, Finke C, Pollak TA. Cognitive impact of neuronal antibodies: encephalitis and beyond. Transl Psychiatry. 2020;10:304.
Kayser C, Fritzler MJ. Autoantibodies in systemic sclerosis: unanswered questions. Front Immunol. 2015;6:167.
Wolin SL, Reinisch KM. The Ro 60 kDa autoantigen comes into focus: interpreting epitope mapping experiments on the basis of structure. Autoimmun Rev. 2006;5:367–72.
Espinosa A, Hennig J, Ambrosi A, Anandapadmanaban M, Abelius MS, Sheng Y, et al. Anti-Ro52 autoantibodies from patients with Sjogren’s syndrome inhibit the Ro52 E3 ligase activity by blocking the E3/E2 interface. J Biol Chem. 2011;286:36478–91.
Reed JH, Gordon TP. Autoimmunity: Ro60-associated RNA takes its toll on disease pathogenesis. Nat Rev Rheumatol. 2016;12:136–8.
Park JW, Kim JH, Kim SE, Jung JH, Jang MK, Park SH, et al. Primary biliary cholangitis and primary sclerosing cholangitis: current knowledge of pathogenesis and therapeutics. Biomedicines. 2022;10:1288.
Arbour L, Rupps R, Field L, Ross P, Erikson A, Henderson H, et al. Characteristics of primary biliary cirrhosis in British Columbia’s First Nations population. Can J Gastroenterol. 2005;19:305–10.
Bourgonje AR, Vogl T, Segal E, Weersma RK. Antibody signatures in inflammatory bowel disease: current developments and future applications. Trends Mol Med. 2022;28:693–705.
Ali F, Rowley M, Jayakrishnan B, Teuber S, Gershwin ME, Mackay IR. Stiff-person syndrome (SPS) and anti-GAD-related CNS degenerations: protean additions to the autoimmune central neuropathies. J Autoimmun. 2011;37:79–87.
Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39.
Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum. 2005;52:3586–95.
Quaden DH, De Winter LM, Somers V. Detection of novel diagnostic antibodies in ankylosing spondylitis: An overview. Autoimmun Rev. 2016;15:820–32.
Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2 + and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–80.
Kronbichler A, Lee KH, Denicolò S, Choi D, Lee H, Ahn D, et al. Immunopathogenesis of ANCA-associated vasculitis. Int J Mol Sci. 2020;21:7319.
Paroli M, Gioia C, Accapezzato D. New insights into pathogenesis and treatment of ANCA-associated vasculitis: autoantibodies and beyond. Antibodies. 2023;12:25.
Sorice M, Misasi R. Different domains of beta(2)-glycoprotein I play a role in autoimmune pathogenesis. Cell Mol Immunol. 2020;17:1210–1.
Weaver JC, Krilis SA, Giannakopoulos B. Oxidative post-translational modification of beta 2-glycoprotein I in the pathophysiology of the anti-phospholipid syndrome. Free Radic Biol Med. 2018;125:98–103.
Jelusic M, Sestan M, Giani T, Cimaz R. New insights and challenges associated with IgA vasculitis and IgA vasculitis with nephritis—is it time to change the paradigm of the most common systemic vasculitis in childhood? Front Pediatrics. 2022;10:853724.
Sestan M, Jelusic M. Diagnostic and management strategies of IgA vasculitis nephritis/henoch-schönlein purpura nephritis in pediatric patients: current perspectives. Pediatr Health, Med Therapeutics. 2023;14:89–98.
Fresquet M, Lockhart-Cairns MP, Rhoden SJ, Jowitt TA, Briggs DC, Baldock C, et al. Structure of PLA2R reveals presentation of the dominant membranous nephropathy epitope and an immunogenic patch. Proc Natl Acad Sci USA. 2022;119:e2202209119.
Seifert L, Hoxha E, Eichhoff AM, Zahner G, Dehde S, Reinhard L, et al. The most N-terminal region of THSD7A is the predominant target for autoimmunity in THSD7A-associated membranous nephropathy. J Am Soc Nephrol. 2018;29:1536–48.
Lohmann T, Hawa M, Leslie RDG, Lane R, Picard J, Londei M. Immune reactivity to glutamic acid decarboxylase 65 in stiff-man syndrome and type 1 diabetes mellitus. Lancet. 2000;356:31–35.
Elvers KT, Geoghegan I, Shoemark DK, Lampasona V, Bingley PJ, Williams AJK. The core cysteines, (C909) of islet antigen-2 and (C945) of islet antigen-2β, are crucial to autoantibody binding in type 1 diabetes. Diabetes. 2013;62:214–22.
Nakayama M, Beilke JN, Jasinski JM, Kobayashi M, Miao D, Li M, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117:1835–43.
Skärstrand H, Lernmark Å, Vaziri-Sani F. Antigenicity and epitope specificity of ZnT8 autoantibodies in type 1 diabetes. Scand J Immunol. 2013;77:21–29.
McLaughlin KA, Richardson CC, Ravishankar A, Brigatti C, Liberati D, Lampasona V, et al. Identification of Tetraspanin-7 as a target of autoantibodies in type 1 diabetes. Diabetes. 2016;65:1690–8.
Nicholes N, Date A, Beaujean P, Hauk P, Kanwar M, Ostermeier M. Modular protein switches derived from antibody mimetic proteins. Protein Eng Des Sel. 2016;29:77–85.
Momin AA, Hameed UFS, Arold ST. Passenger sequences can promote interlaced dimers in a common variant of the maltose-binding protein. Sci Rep. 2019;9:20396.
Weil MT, Mobius W, Winkler A, Ruhwedel T, Wrzos C, Romanelli E, et al. Loss of myelin basic protein function triggers myelin breakdown in models of demyelinating diseases. Cell Rep. 2016;16:314–22.
van Delft MAM, Huizinga TWJ. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun. 2020;110:102392.
Kongkaew S, Yotmanee P, Rungrotmongkol T, Kaiyawet N, Meeprasert A, Kaburaki T, et al. Molecular dynamics simulation reveals the selective binding of human leukocyte antigen alleles associated with behcet’s disease. PLoS One. 2015;10:e0135575.
Hu CJ, Pan JB, Song G, Wen XT, Wu ZY, Chen S, et al. Identification of novel biomarkers for Behcet disease diagnosis using human proteome microarray approach. Mol Cell Proteom. 2017;16:147–56.
Takeno M. The association of Behçet’s syndrome with HLA-B51 as understood in 2021. Curr Opin Rheumatol. 2022;34:4–9.
Eriksson D, Royrvik EC, Aranda-Guillen M, Berger AH, Landegren N, Artaza H, et al. GWAS for autoimmune Addison’s disease identifies multiple risk loci and highlights AIRE in disease susceptibility. Nat Commun. 2021;12:959.
Pallan PS, Wang C, Lei L, Yoshimoto FK, Auchus RJ, Waterman MR, et al. Human cytochrome P450 21A2, the major steroid 21-hydroxylase: structure of the enzyme· progesterone substrate complex and rate-limiting c–h bond cleavage. J Biol Chem. 2015;290:13128–43.
Seissler J, Schott M, Steinbrenner H, Peterson P, Scherbaum W. Autoantibodies to adrenal cytochrome P450 antigens in isolated Addison’s disease and autoimmune polyendocrine syndrome type II. Exp Clin Endocrinol Diabetes. 1999;107:208–13.
Takizawa S, Endo T, Wanjia X, Tanaka S, Takahashi M, Kobayashi T. HSP 10 is a new autoantigen in both autoimmune pancreatitis and fulminant type 1 diabetes. Biochem Biophys Res Commun. 2009;386:192–6.
Yokode M, Shiokawa M, Kodama Y. Review of diagnostic biomarkers in autoimmune pancreatitis: where are we now? Diagnostics. 2021;11:770.
Wang KL, Tao M, Wei TJ, Wei R. Pancreatic beta cell regeneration induced by clinical and preclinical agents. World J Stem Cells. 2021;13:64–77.
Download references
Acknowledgements
This work was supported by CAS Strategic Priority Research Program (XDB37030103 to HEX); Shanghai Municipal Science and Technology Major Project (2019SHZDZX02 to HEX); Shanghai Municipal Science and Technology Major Project (HEX); The National Natural Science Foundation of China (32130022, 82121005); the Lingang Laboratory Grant (LG-GG-202204-01 to HEX); the National Key R&D Program of China (2018YFA0507002 to HEX). Figures were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License ( https://creativecommons.org/licenses/by/3.0/ )
Author information
Authors and affiliations.
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China
Shu-jie Li, Yan-li Wu, Juan-hua Chen, Shi-yi Shen, Jia Duan & H. Eric Xu
Department of Traditional Chinese Medicine, Fujian Medical University Union Hospital, Fuzhou, 350000, China
University of Chinese Academy of Sciences, Beijing, 100049, China
Shi-yi Shen & Jia Duan
Zhongshan Institute for Drug Discovery, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Zhongshan, 528400, China
School of Life Science and Technology, Shanghai Tech University, Shanghai, 201210, China
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Shu-jie Li , Jia Duan or H. Eric Xu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Li, Sj., Wu, Yl., Chen, Jh. et al. Autoimmune diseases: targets, biology, and drug discovery. Acta Pharmacol Sin 45 , 674–685 (2024). https://doi.org/10.1038/s41401-023-01207-2
Download citation
Received : 22 March 2023
Accepted : 20 November 2023
Published : 14 December 2023
Issue Date : April 2024
DOI : https://doi.org/10.1038/s41401-023-01207-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- autoimmune disease
- autoantigen
- immunotherapy
- type 1 diabetes
- drug discovery
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
Autoimmune diseases are a pathophysiological state wherein immune responses are directed against, and damage, the body's own tissues (autoimmunity). Common examples of autoimmune disease include ...
Autoimmune diseases have been shown to affect 3-5% of the population and become one of the most important public health problems. 5,6 Recently, Conrad et al. reported a population-based cohort ...
Despite extensive research, there are no genetic tools that can be used clinically to predict the risk of autoimmune disease. Indeed, the concordance of autoimmune disease in identical twins is 12-67%, highlighting not only a role for environmental factors, but also the potential importance of stochastic or epigenetic phenomena.
Autoimmune diseases have been shown to affect 3-5% of the population and become one of the most important public health problems. 5,6 Recently, Conrad et al. reported a population-based cohort study of 19 autoimmune diseases in the UK about 22,009,375 individuals from 2000 to 2019. 7 During this period, 978,872 individuals were newly ...
Schematic representation of anti CD 20 targeted immunotherapy in autoimmune diseases. Rituximab (RTX), a genetically engineered chimeric monoclonal antibody with specific affinity to CD 20 surface antigen on B cells, intercepts two major functions, the cell cycle progression and differentiation of B cells. 13 Being a B cell depleting agent, it is considered as beneficial or even curative by ...
A recent review of NIH's autoimmune disease research activities by the National Academies of Sciences, Engineering, and Medicine has found a number of gaps and limitations in the approaches that the different NIH institutes pursue, and among their recommendations was the creation of an Office of Autoimmune Disease/Autoimmunity Research within ...
The Research Topic: Insights in Autoimmune and Autoinflammatory Disorders: 2021 was implemented to highlight latest advancements in research across the field of Immunology in Autoimmunity and Autoinflammation by discussing recent advances, current challenges, and future perspectives. Under the Research Topic 13 articles were published, 10 contributions highlight topics relevant to autoimmune ...
Autoimmune diseases (AIDs) arise from a breakdown in immunological self-tolerance, wherein the adaptive immune system mistakenly attacks healthy cells, tissues and organs. ... Research articles ...
Autoimmune Diseases publishes original research articles and review articles on all aspects of autoimmunity. Articles focus on the basic biology and mechanism of the disease, and medical treatment of autoimmune diseases. ... Research Article. Open access. Symptoms and Severity of COVID‐19 in Patients with Immune‐Mediated Inflammatory ...
The increasing prevalence of autoimmune diseases has become one of the leading concerns in public health.1,2 Autoimmune diseases occur when the immune system mistakenly attacks healthy cells and tissues in the body, leading to chronic inflammation, tissue damage, and organ dysfunction.1 As more people are diagnosed with autoimmune disease, the burden on health-care systems, families, and ...