Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 May 2021

Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources
- Manyou Yu 1 ,
- Irene Gouvinhas 1 ,
- João Rocha 2 &
- Ana I. R. N. A. Barros 1 , 3
Scientific Reports volume 11 , Article number: 10041 ( 2021 ) Cite this article
35k Accesses
158 Citations
1 Altmetric
Metrics details
- Biochemistry
Plants with medicinal properties play an increasingly important role in food and pharmaceutical industries for their functions on disease prevention and treatment. This study characterizes the phenolic composition and antioxidant activity of seven medicinal and food plants, including the leaves of Salvia officinalis L., Rosmarinus officinalis L., Olea europaea L., and Punica granatum L., as well as the leaves and young stems of Ruta graveolens L., Mentha piperita L., and Petroselinum crispum , Mill., by using colorimetric, chromatographic, and spectrophotometric assays. Results revealed that the hydro-methanolic leaf extracts of P. granatum (pomegranate) displayed the highest content of total phenols (199.26 mg gallic acid per gram of plant dry weight), ortho -diphenols (391.76 mg gallic acid per gram of plant dry weight), and tannins (99.20 mg epicatechin per gram of plant dry weight), besides a higher content of flavonoids (24 mg catechin per gram of plant dry weight). The highest antioxidant capacity measured by ABTS, DPPH, and FRAP (2.14, 2.27, and 2.33 mM Trolox per gram of plant dry weight, respectively) methods was also obtained in pomegranate leaf extracts, being 4–200 times higher than the other species. Such potent antioxidant activity of pomegranate leaves can be ascribed to the presence of different types of phenolic compounds and the high content in tannins, whilst phenolic acids and flavonoids were found to be the dominant phenolic classes of the other six plants. Consequently, despite the well-known antioxidant properties of these plant species, our study suggests pomegranate leaf can stand out as a relatively more valuable plant source of natural bioactive molecules for developing novel functional food-pharma ingredients, with potential for not only promoting human health but also improving bio-valorization and environment.
Similar content being viewed by others

Comparison of the content of active ingredients and antioxidant, antibacterial and anti-inflammatory activities in different parts of Fructus Choerospondiatis

Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling
Comprehensive profiling of phenolic compounds and triterpenoid saponins from Acanthopanax senticosus and their antioxidant, α -glucosidase inhibitory activities
Introduction.
The recent development of functional foods and pharmaceutical products based on medicinal and food (namely fruits and vegetables) plants has brought improvements to all aspects of life, including the alleviation of physical disorders, the reduction in the use of synthetic antibiotics, and the increase in life expectancy 1 , 2 . Indeed, these plants have long been used as safe, effective and sustainable sources of natural antioxidants or free radical scavengers, particularly phenolic compounds, such as phenolic acids, flavonoids, tannins, stilbenes, and anthocyanins 2 . Those phenolics are mostly regarded to confer upon the antioxidant activity of medicinal and food plants, making a marked contribution in the fight against many pathological conditions such as cancer, diabetes, aging, cardiovascular, and other degenerative diseases 2 , 3 , 4 , 5 .
Salvia officinalis L., Rosmarinus officinalis L., and Mentha piperita L. commonly named as sage, rosemary, and peppermint, respectively, belongs to the family of Lamiaceae. They are well-known herbs and spices used in foods for flavors and aromas. Infusions, leaves or essential oils of its each species are reported to possess therapeutics in anti-cancer, anti-microbial, anti-diabetes, and gastrointestinal diseases, etc. 3 , 6 , 7 , 8 . Several bioactivities of sage like antinociceptive, hypolipidemic, and memory-enhancing effects have been demonstrated with clinical trials 7 . Rosmarinic acid is abundant both in sage and rosemary, contributing to their anti-inflammatory properties 3 , 6 , 7 . Flavonoids, phenolic lignans and stilbenes, and essential oils are expected to be responsible for the aroma effects of peppermint 8 .
Rue ( Ruta graveolens L.) has been one of the key plants of the European pharmacopoeia since ancient times for the use in tremors, paralysis, nervine disorders, and joint pain 9 . And nowadays, it becomes medicine in Mediterranean region, due to its prominent biological activities, especially neuroprotection 9 , 10 . Rutin, psoralen, limonene, and pinene are reported as main constituents in this plant extracts or rue oils 9 , 10 .
Olive ( Olea europaea L.) oil is one of the major components of the Mediterranean diets. Recently, phenolics present in olive leaves, especially the oleuropein, are reviewed to be potential economic and renewable source of natural by-products, attributed to its antioxidant, antihypertensive, hypoglycemic, hypocholesterolemic and cardioprotective activity 11 , 12 .
Parsley ( Petroselinum crispum Mill.), used as culinary and medicinal herb, is originated from Mediterranean region. Phytochemicals particularly apigenin, coumarins, myristicin, and apiol are active compounds rich in parsley leaves, exhibiting diverse pharmacological properties, such as cyto-, gastro-, brain-, nephron-protective effects, and so on 13 , 14 , 15 .
Pomegranate ( Punica granutum L.) a deciduous shrub in the family of Lythraceae, is one of the oldest known plants. Both the edible (namely fruit juice) and non-edible parts (including seeds, peels, leaves, roots and bark) of this plant have been evidenced to have a wide range of health benefits, largely resulting from its abundant phenolic acid, flavonoids, tannins, amino acids, and alkaloids 16 , 17 . However, the importance of pomegranate leaves, as agricultural and industrial waste, is of great interest and value to be emphasized by means of describing its beneficial effects and studies performed on this field.
Within the frame, materials from the seven medicinal and food plants aforementioned, that is, leaves and young stems (easy for picking) of rue, peppermint, and parsley, as well as the leaves of sage, rosemary, olive, and pomegranate are outstanding for their higher levels of phenolic contents and antioxidant capacities, along with relatively lower (dose-dependent) or inexistent toxicity 6 , 7 , 8 , 9 , 11 , 13 , 15 , 17 . Therefore, in an attempt to explore plant-based alternative solutions in promoting health, as well as paving the way towards our future pre-clinical and clinical studies, we aimed to analyze the phenolic classes (total phenols, ortho -diphenols, flavonoids, and tannins) and antioxidant activities of different plant species under the same evaluation condition. Furthermore, the principal phenolic constituents were chromatographically characterized to investigate the relationship between the phenolic content and antioxidant activity.
Results and discussion
Phenolic content of tested medicinal and food plants.
Results of colorimetric and spectrophotometric analysis of seven medicinal and food plants were showed in Table 1 . In general, the total phenolic content of the selected plant species was found to be at the highest level in pomegranate leaf extracts at 199.26 mg of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW), followed by three Lamiaceae species, including peppermint (70.06 mg GAE g −1 DW), sage (50.89 mg GAE g −1 DW) and rosemary (48.48 mg GAE g −1 DW). On the contrary, parsley displayed the lowest value of total phenols (6.94 mg GAE g −1 DW). The same trend was observed concerning the content of ortho -diphenols and tannins of all investigated samples, reporting the following sequence: pomegranate > peppermint > sage > rosemary > rue > olive > parsley. The ortho -diphenol and tannin content of the methanolic extracts ranged from 26.40 to 391.76 mg GAE g −1 DW, and from 1.33 to 99.20 mg of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW), respectively. Moreover, results on total flavonoids content showed a different pattern compared to other phenolic classes, with peppermint showing maximum values at 70.14 mg of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW), following with rosemary (49.14 mg CATE g −1 ), sage (43.92 mg CATE g −1 ), and pomegranate (24.34 mg CATE g −1 ). Furthermore, the flavonoid content of olive leaf was higher than that of rue, in contrast to the trend of the other phenolic classes. Rosemary and sage had comparatively high levels of flavonoids, while the minimum values were reported for parsley.
Different phenolic contents of different plant samples have been reported in the literature 12 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 . For instance, the total phenol content of sage and peppermint was 27.94 and 45.25 mg GAE 100 g −1 DW, meanwhile the flavonoid content of them was 27.54 and 25.17 mg catechin per 100 g, which were much lower than that of our results 19 . Parsley extracts had 1.583 GAE mL −1 of total phenols, 0.091 mg catechin mL −1 of flavonoids, and 1.167 mg catechin mL −1 of condensed tannins 26 . Salama et al. 12 described significant differences in the amounts of total phenolics, flavonoids, and tannins of olive leaves, under different extraction solvents, ranging from 42.02 to 85.50 mg GAE g −1 , 31.22 to 105.19 mg quercetin g −1 , and 30.92 to 51.03 mg tannic acid g −1 , respectively. The contents of phenolic and flavonoid compounds in rue were 14.1 GAE g −1 and 15.8 mg rutin g −1 of dry extracts 20 . Some studies 27 , 28 , 29 have evidenced considerably high level of phenolics in pomegranate leaf extracts, up to 328 mg GAE g −1 DW. Interestingly, pomegranate leaves are characterized by carbohydrates, reducing sugars, sterols, saponins, flavonoids, ellagitannins, piperidine alkaloids, flavones, glycosidic compounds, which are the richest source of phytochemicals when considering the non-edible parts of this species, some food products (red wine, green tea, etc.), and another 109 medicinal plants 30 , 31 , 32 . Our results disclosed that tannins were the main phenolic compounds of pomegranate leaf extract, which has also been corroborated by other studies 33 .
As shown in data (Table 1 ), significant differences ( p < 0.001) around 29, 15, 92 and 75 times were observable respectively for total phenols, ortho -diphenols, flavonoids and tannins in the seven plant extracts, indicating that each phenolic classes exhibited considerably different content among the studied plants. This result was in agreement with other authors 34 , who found that depending on the plant species and botanical family, strong differences were found among 10 medicinal herbs and 11 spices. Meanwhile, the same authors 34 observed a wide variance of phenolics in different samples of the same species, such as the total phenolic content of nine independent samples of peppermint was from 18.3 to 284.3 mg GAE g −1 . Moreover, contents of total phenolics, flavonoids, and condensed tannins of 13 different provenances of rosemary, collected in different seasons ranged from 22.46 to 44.57 mg GAE g −1 DW, from 1.49 to 5.01 mg quercetin g −1 , and from 0.81 to 1.71 mg CATE g −1 DW, respectively 18 . Our results showed inconsistency with this observation, probably attributed to the varieties, or geographical differences, as well as to the collection time, agroclimatic conditions and other relevant factors 24 , 25 . However, to some extent, pomegranate leaf was supposed to have a relatively higher phenolic content than many other medicinal plants. Therefore, it can be inferred that pomegranate leaf could be an important valuable source of bioactive compounds for medicinal purposes and health care.
In addition, in the current study, the colorimetric analysis of flavonoids varied between pomegranate leaf (orange-yellowish) with other plants (pink) and the standard (catechin, pink) under the same conditions (as below described in the methods). This visual observation may be related to the fact that leaves from pomegranate have different predominant sub-classes of flavonoids, different from that existing in the other studied plants 32 . So, the methodology, especially to normalize the use of standards such as quercetin or rutin 35 should be modified to accurately quantify the amount of flavonoids.
In vitro antioxidant activity
The in vitro antioxidant activity assays were carried out to assess the capacity of plant extracts to scavenge free radicals including 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid radical cation (ABTS +· ) and 2,2‐di(4‐tert‐octylphenyl)‐1‐picrylhydrazyl radical (DPPH·), as well as the ability to reduce ferric (III) iron to ferrous (II) iron. Overall, Table 1 revealed that all the species displayed high antioxidant capacities, although significant differences were observed ( p < 0.001), ranging from 0.01 to 2.14 mM Trolox per gram of plant dry weight (mM Trolox g −1 ) for ABTS, from 0.01 to 2.27 mM Trolox g −1 for DPPH, and from 0.01 to 2.33 mM Trolox g −1 for FRAP (ferric reducing antioxidant power), with large variation over 210-fold. It was found that pomegranate always exhibited the highest antioxidant properties (2.14–2.33 mM Trolox g −1 ) throughout the three measurements, followed by peppermint (0.35–0.50 mM Trolox g −1 ), sage (0.27–0.40 mM Trolox g −1 ), rosemary (0.27–0.42 mM Trolox g −1 ), rue (0.10–0.16 mM Trolox g −1 ), and olive leaf (0.11–0.15 mM Trolox g −1 ). No significant difference was observed between sage and rosemary, and between rue and olive leaf. However, parsley extracts reported the lowest antioxidant potential (0.01 mM Trolox g −1 ).
Previous data regarding the antioxidant capacities of sage, rosemary, rue, olive leaf, peppermint, parsley, and pomegranate leaf have been reported by several authors 12 , 14 , 18 , 22 , 26 , 31 , 36 . The IC 50 values of ABTS and DPPH radical scavenging activity, as well as the EC 50 values of reducing powder regarding olive leaves ranged from 20.13 to 190.95 µg mL −1 , from 17.97 to 41.64 µg mL −1 , and from 90 to 216 µg mL −1 , arising from diverse extraction solvents 12 . Rosemary leaves displayed 75.04 and 9.08 µg mL −1 of IC 50 by ABTS and DPPH assay, along with 4.12 µM by FRAP method 18 . Farnad et al. 22 reported the methanol-ethanol (1:1) extract of peppermint had the best DPPH radical scavenging ability (10.05 mg mL −1 of IC 50 ) and ferric reducing power (184.22 µmol per 100 g powder). The ethanolic extract of parsley displayed 0.34 mg AAE mL −1 (milligrams of ascorbic acid equivalents per milliliter) of DPPH and 0.942 mg AAE mL −1 of FRAP, which was correlated with the anti-glycation activity of this extract 26 . The best antioxidant capacities conducted by DPPH (17.09% of IC 50 ) and FRAP (458.26 mmol Fe II L −1 ) were determined for sage leaves which were collected in May 36 . Cefali et al. 14 stated the rue extracts exhibited antioxidant potential against DPPH (281.02 µg mL −1 of IC 50 ) and ABTS (587.98 µg mL −1 of IC 50 ) radicals, indicating the premature aging protective effect.
Importantly, several studies in vitro and in vivo have recorded the superior antioxidant capacity of pomegranate leaves by contrast with its non-edible parts, of which leaves are as effective as peels in the anti-bacterial, analgesic, acute and chronic anti-inflammatory effects 37 , 38 , while more potent than flowers, stems, and seeds 31 , 39 , 40 , 41 , 42 . Authors proved the potency of pomegranate leaf was higher than that of flower in the prevention of ethylene glycol-induced nephrolithiasis, in the inhibition of DPPH and hydroxyl radicals, and in the reduction of ferric iron 39 , 40 . Data 41 highlighted leaves worked more effectively than stems and led to the most loss of MMP (mitochondrial membrane permeability) potential, consequently suggested as an anti-cancer and anti-proliferative agent. Elfalleh et al. 31 illustrated the highest reducing power (348.68 µg mL −1 of EC 50 ) occurring in the aqueous extract of pomegranate leaf. Furthermore, a higher antioxidant and enzyme inhibitory activity was exposed in two extracts (methanolic and water) of pomegranate leaves among different fruit tree leaves 28 . The ethanolic extracts of pomegranate leaf also exhibited remarkable antioxidant and anti-glycation ability of twenty edible and medicinal plants 29 . The level of anti-radical and ferric reducing properties of pomegranate leaves in our results was similar to some authors 42 . However, comprehensively comparative research involving in the phytochemical and antioxidant properties between pomegranate leaves and other numerous medicinal plants is still scarce; Widely practical application of pomegranate leaf hasn’t come into being, although different biological activities of this material extracts are studied increasingly. Many authors have deeply reviewed for sage, rosemary, peppermint, rue, parsley, and olive leaf. Thus it is worth stressing on the brilliant phenolics and antioxidant property of pomegranate leaves, and developing high added-value products from these materials in the food, pharmaceutical, or even nutraceutical and cosmeceutical industries.
Chromatographic analysis of phenolic compounds
With the development of chromatographic techniques, the phenolic chemistry of many plants has been explored and analyzed to a certain degree, providing us important reference data. To obtain a more complete picture of the quality and quantity of phenolic constituents in the selected plants, 64 phenolic compounds were identified (Table 2 ), of which 59 were quantified with authentic standards relying on RP-HPLC-DAD, as well as by comparison with the literature (retention time, UV/Visible λ max , and spectra). Concentrations of identified phenolics were expressed as milligram per gram dry weight of plant (mg g −1 ).
As shown in Table 2 and Fig. S1 , phenolic profile of diverse plants was significantly different. Leaf extracts of both sage and rosemary were characterized by a high proportion of rosmarinic acid (4.61 mg g −1 or 4.31 mg g −1 , respectively). Rue presented the highest content of rutin (26.10 mg g −1 ), followed by epicatechin gallate (7.82 mg g −1 ). The major phenolic components in olive leaves were oleuropein and its derivatives. Flavanones, especially eriodictyol glycosides, following rutin were found as predominant in the leaf and stem extracts of peppermint. Parsley was described in high amount of apigenin-7- O -apiosylglucoside also called apiin (4.04 mg g −1 ) and epicatechin (3.72 mg g −1 ) in its leaf and stem extracts. The principal phenolic constituents in pomegranate leaves were hydrolyzable tannins, particularly ellagitannin I (56.06 mg g −1 ) and ellagitannin II (45.16 mg g −1 ), ranking the highest concentrations among all identified compounds.
On the other hand, results from Table 2 and Fig. S1 also showed that the most abundant phenolic classes in the tested samples were phenolic acids, flavonoids, tannins, and phenylethanoids. A considerable variation of phenolics was found, ranging, for instance, from 0.03 mg g −1 of 2,3-hydrocybenzoic acid to 56.06 mg g −1 of ellagitannin I. For each identified compound, significant differences were observed ( p < 0.05), such as gallocatechin. The most widespread phenolic acids present in the studied samples included hydroxybenzoic acids (gallic acid and its derivative, vanillic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, and neochlorogenic acid), and their ester derivatives (e.g. rosmarinic acid). Significantly high contents of chlorogenic acid (1.54 mg g −1 ) and neochlorogenic acid (1.96 mg g −1 ), and the presence of coumaric acid were perceptible in rue extracts. Ellagic acid and its derivatives were abundant in pomegranate leaves. Except in sage and rosemary, rosmarinic acid was also found in peppermint (0.23 mg g −1 ), but its concentration was lower than that in literature 43 . The special existence of rosmarinic acid, rosmanol, epirosmanol, carnosol, and carnosic acid in sage and rosemary was consistent with other authors 23 , 25 , 44 , 45 .
Besides flavanols including gallocatechin, catechin and epicatechin gallate, then various flavones (luteolin and apigenin) and flavonols (quercetin and diosmetin), mainly in the forms of their derivatives were widely distributed in the most of the studied species. Among them, the highest content of gallocatechin (2.10 mg g −1 ), catechin (3.61 mg g −1 ) and epicatechin gallate (7.82 mg g −1 ) was detected in parsley, rosemary, and rue, respectively. Epicatechin was only found in parsley with a good quantity (3.72 mg g −1 ). Furthermore, the main flavonoids from our data present in sage, rosemary, rue, peppermint, and parsley were apigenin glycosides, luteolin glycosides, quercetin glycosides, flavanone glycosides, and apigenin glycosides, respectively. In addition, peppermint also had comparative amounts of luteolin and quercetin glycosides. Likewise, pomegranate leaves possessed several apigenin and luteolin glycosides. Particularly, rutin presented the highest proportion (26.10 mg g −1 ) in rue, followed by peppermint (9.90 mg g −1 ), while the lowest (0.86 mg g −1 ) in olive leaf.
An important observation is that pomegranate leaf extracts held the greatest number of hydrolyzable tannins, especially ellagitannins. Nevertheless, no ellagitannins were detected by the HPLC method in the other six plants, while condensed tannins were present by the spectrophotometric approach. This was possibly caused by the lack of authentic standards involving different tannins, which need to be performed in the chromatographic analysis. In practice, certain studies have reported the tannins present in sage, rosemary, peppermint, rue, parsley, and olive leaves 12 , 18 , 19 , 26 , 46 , 47 , 48 , mainly in the form of condensed tannins.
In some cases, phenylethanoids, which are phenethyl alcohol-structured phenolic antioxidants, were abundantly found in olive leaves, including oleuropein and its derivatives, followed by tyrosol and verbascoside. These molecules may conduct to its high antioxidant properties 11 , 49 . Tyrosol existed in highest concentration in rosemary (4.56 mg g −1 ), but in small quantity in olive leaf (0.75 mg g −1 ) and rue (0.19 mg g −1 ).
Many studies have described the domination of rosmarinic acid in sage and rosemary, detected in varied amounts depending on phenophase, genotypes, extraction methods, and geographical conditions 23 , 24 , 25 , 36 , 44 , 45 , 50 . A concentration ranging from 0.27 to 2.49% of rosmarinic acid was determined in rosemary leaf extract, according to regions 44 . Khaleel et al. 45 reported 4.5 µg mL −1 of rosmarinic acid in aqueous extract of rosemary, whereas 17.3 µg mL −1 was measured in our methanolic extract of this plant. Exceptionally high content of rosmarinic acid was found in May extract (19.375 mg L −1 ) of sage leaves described by Generalić et al. 36 , very close to our data (18.653 mg L −1 ). Roby et al. 23 declared that the predominant phenolic compounds in sage methanolic extract were ferulic acid (18%), rosmarinic acid (17%) and apigenin (14%) of the total extracted phenols, while in our results, rosmarinic acid and apigenin glycoside III were primary and accounted for 9% and 13% of the total phenolics of sage. Among more than one-hundred active ingredients of rue, rutin, as one of its major compounds, has been a topic of interest for researchers 9 , 20 . Asgharian et al. 20 detected a high level of rutin (40.15 mg g −1 ) by extraction with 70% ethanol, which was higher than that of our study. Melnyk et al. 46 identified rutin as the highest content of phenolics in the rue methanolic extract, consistent with the present work. Several studies 48 , 49 have reported oleuropein and its derivatives as the dominant phenolics in the olive leaf, according with our results. As shown in data (Table 2 and Fig. S1 ), up to 20 phenolic compounds were identified in methanolic extract of olive leaf, more than those identified in other six plants, evidencing it as a rich source of bioactive compounds. However, the composition of olive leaf shows a remarkable variability due to location, climatic-seasonal factors, and cultivation practices, suggesting a trend to understand the factors that control the composition of olive leaves. This can be worthy for the harvesting and production of suitable extracts to be applied in human health. Kapp et al. 43 demonstrated eriocitrin, as a powerful bioactive compound, was the most abundant phenolics in peppermint, in accord with our records, composed of 38% of its aqueous extract, or reaching from 19.9 to 68.1% in 26 peppermint tea samples, respectively. However, the same authors 43 reported that rosmarinic acid accounted for a highest proportion (54.2%) of phenolics in one peppermint tea sample which was originated from Estonia. Additionally, other authors 22 , 47 , 51 also pointed out different dominant phenolics in peppermint, such as epicatechin, naringenin, caffeic acid, chlorogenic acid, 4-hydroxybenzoic acid, which can be attributed to diverse varieties, growing environment, and extraction conditions. The main finding of the present work performed on parsley corresponded to several studies 15 , 52 that apiin extractability was maximum when the solvent was ethanol, methanol or acetone. Yet Hozayen et al. 53 and Aissani et al. 21 conducted rosmarinic acid and quinic acid as the most abounded constituent in aqueous and methanol extracts of parsley, respectively. Fourteen phenolic constituents (Figure S1 ) of pomegranate leaf extracts were preliminarily identified and quantified by reference to chromatographic parameters and the literature. These results are agreeable to other researchers 33 , 54 , 55 , 56 , highlighting that ellagic acid and its derivatives, ellagitannins (punicalin, granatin A and B, etc.), flavone (apigenin, luteolin) and its glycosides, and flavonol (kaempferol) and its glycosides, are the principal phenolics in pomegranate leaves. In addition, many ellagitannins (such as punicalagins, punicafolin, castalagin, corilagin, strictinin, tercatain, brevifolin), and their galloyl and/or hexahydroxydiphenoyl (HHDP) substitutions, have been isolated from the leaf 57 . Other flavonoid derivatives like kaempferol, gossypin, quercetin, and rutin were also detected as major constituents in hydro-methanolic or hydro-ethanolic leaf extracts of pomegranate leaves 33 , 57 . However, the detailed structures of tannins and flavonoids of pomegranate leaf will require further identification by mass spectrometry and nuclear magnetic resonance spectroscopy.
Correlation analysis
In order to better understand the relationship between the antioxidant activity (by ABTS, DPPH, FRAP assays) and the phenolic composition (total phenols, ortho -diphenols, flavonoids, tannins) of the studied plants, correlation coefficients ( r ) were determined (Fig. 1 ). Strong relationships were characterized between antioxidant capacities with total phenols and ortho -diphenols (Fig. 1 a,b), indicating that phenolic compounds contribute to the inhibition of oxidative processes. The content of tannins was well correlated with antioxidant potential (Fig. 1 d). No correlation of antioxidant activities was found with flavonoid content (Fig. 1 c). However, a better relationship of flavonoids (Fig. 1 e) or tannins (Fig. 1 f) can be obtained with the antioxidant activity if excluding pomegranate or peppermint from the data, respectively. The above analysis demonstrated that the antioxidant potential from different plants was dependent on both the concentrations and the structures of phenolic compounds, in line with Cai et al. 30 . Compared to radical scavenging assays (ABTS and DPPH), the stronger correlation between reducing power and phenolic contents confirmed that FRAP was more closely related to total phenols, ortho -diphenols and tannins, which was also mentioned by Li et al. 1 .
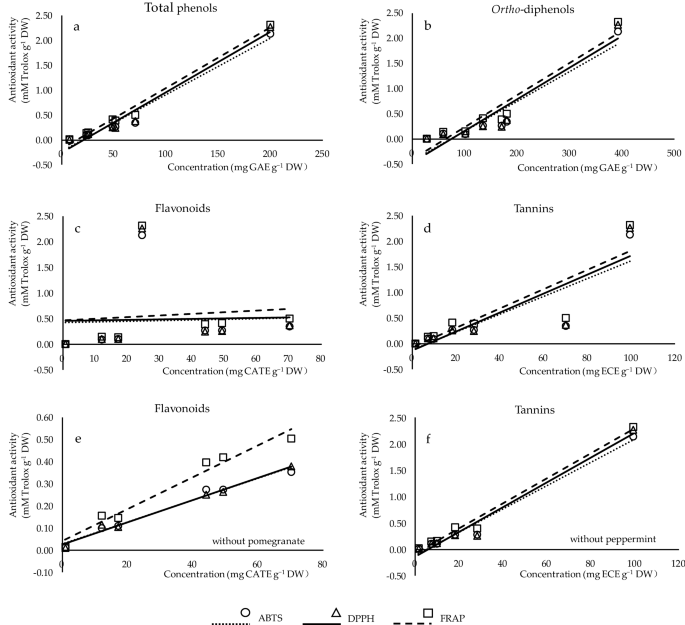
Correlation analysis between the contents of phenolic classes (x-axis) and antioxidant capacities (y-axis) measured by ABTS (circles), DPPH (triangles), and FRAP (squares). ( a – d ) The correlation of total phenols ( r ABTS , DPPH , FRAP = 0.985***, 0.984***, 0.993***), ortho -diphenols ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**), flavonoids ( r ABTS , DPPH , FRAP = 0.038, 0.031, 0.098), and tannins ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**) of the studied plants with their antioxidant activity, respectively. ( e ) The correlation of flavonoids ( r ABTS , DPPH , FRAP = 0.989***, 0.992***, 0.983***) of studied plants excluding pomegranate with their antioxidant activity. ( f ) The correlation of tannins of studied plants excluding peppermint ( r ABTS , DPPH , FRAP = 0.989***, 0.987***, 0.993***) with their antioxidant activity.
There is a highly correlation between the phenolic composition and antioxidant properties of plants. High anti-radical activity of rosemary leaf in summer was strongly related to high amounts of total phenols, total flavonoids, condensed tannins, and carnosic acid 18 . It is suggested that intraperitoneal of hydroalcoholic extract of rue increased serum and brain antioxidant capacity, due to their potent antioxidant activities of total phenolic and flavonoids content, especially rutin, caffeic acid, and apigenin 20 . Parsley methanolic extract inhibited human glioblastima cancer and oxidative stress owing to its antioxidant properties primarily related to phenolic content 21 . Peppermint extracted by various alcoholic solvents are found to have different levels of antioxidant potential, attributed to the presence of vast flavonoids, anthocyanins, and total phenols 22 . The strong reducing power, free radical scavenging capacity, and the inhibition of hydro-peroxide radicals activity of sage leaves can be linked to the high quantity of phenolic acids, especially rosmarinic acid, and certain flavonoids like catechins and flavanols 36 . Makowska-Wąs et al. 49 revealed considerable antioxidant and cytotoxic properties of olive leaf against several human cancers, largely concerned in the existence of phenolic acids, flavonoids, oleuropein, fatty acids, and volatile oils. The high concentration of phenolic components in pomegranate leaf extracts such as tannins, flavonoids, phyto-steroids, terpenoids, and saponins can be responsible for its high antioxidant activity in vitro and in vivo 27 , 28 , 29 , 32 , 58 .
To date, amount of studies have reported the close relationship not only between the phenolic contents but also between the phenolic structures and the antioxidant capacities 28 , 30 , 59 . The level of antioxidant potential of plants mainly depends on the presence and hydroxyl groups of (poly)phenolic compounds. Specifically, the antioxidant ability of phenolic acids is firstly related to the number and position of phenolic hydroxyls, and secondly to the methoxy and carboxylic acid groups 59 . Rosmarinic acid which was mainly detected in sage, rosemary, and peppermint in our work, is an ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid, comprising two catechol moieties, thus having two pairs of ortho hydroxyl groups grafted on two phenolic rings 18 . Gallic and chlorogenic acid are well-known antioxidant agents, due to three and two active hydroxyl groups on the aromatic ring, respectively 59 . Moreover, the catechol structure in the B-ring, the 2,3-double bond conjugated to a 4-oxo functionality, and the available of both 3- and 5-hydroxyl groups of flavonoids are essential for assessing their antioxidant properties 28 . Rutin is a rutinoside of quercetin with one of the four hydroxyl groups at position C-3 substituted with glucose and rhamnose sugar groups 20 . Apiin or eriocitrin is a apigenin or eriodictyol glycoside, on which the different glycoside moiety is located at position C-7 via a glycosidic linkage along with two or three residual hydroxyl groups on the phenolic rings 15 , 43 . Furthermore, phenylethanoids are characterized by a phenethyl alcohol (C6–C2) moiety attached to a β-glucopyranose/β-allopyranose via a glycosidic bond. Studies indicated the ortho -dihydroxyphenyl groups were the most significant, and the steric hindrance, the number and the position of phenolic hydroxyls were also thought to play an important role 60 . Oleuropein with two hydroxyl groups is an ester of elenolic acid and hydroxytyrosol, and has a oleosidic skeleton that is common to the secoiridoid glucosides of Oleaceae 49 . The strong correlation of antioxidant property with well-identified phenolic acids, flavonoids, and oleuropein present in sage, rosemary, peppermint, rue, parsley, and olive leaves has been individually demonstrated to explain their diverse biological functions 6 , 7 , 8 , 9 , 11 , 13 . In addition, ellagic acid and tannins, defined as polyphenols, are complex chemical substances, possessing plentiful hydroxyl groups, especially ortho -dihydroxyl or galloyl groups 61 . Bigger tannin molecules appear more galloyl and ortho -dihydroxyl groups, consequently, their activities are stronger 61 . Ellagitannins, ellagic acid, and their metabolites have been reported to exhibit numerous beneficial effects on human health including antioxidant, anti-inflammatory, anti-cancer, prebiotic, and cardio-protective properties 61 . Thus they deserve to be part of a healthy diet as functional foods.
The researches on the structure–activity relationship between phenolics and their antioxidant activities have focused on phenolic acids and flavonoids, as well as oleuropein and its derivatives owing to their partially acknowledged health-promoting effects 2 , 30 . However, the benefits of medicinal and food plants may arise from the action of some less well-studied antioxidant molecules or from a synergy of certain antioxidants 30 . Cai et al. 30 found some anticancer-related medicinal plants contained higher quantities and more sorts of tannins, quinones, phenolic terpenoids and special phenolic glycosides than that of phenolic acids and flavonoids. Regarding pomegranate leaves, some authors detected kaempferol 54 or kaempferol 3- O -glycoside 33 as the main compound in ethanolic extracts, while others found as ellagic acid 55 . The principal ellagitannins of pomegranate leaves also differed from one another, considered as granatin B 56 , or castalagin derivative 33 , or undefined galloy-HHDP derivatives 55 . This difference may be induced by varieties, phenology, and growing conditions. In our study, the potent antioxidant capacity of pomegranate leaves was highly correlated with the content of tannins, which can be considered as the key antioxidant contributors of this plant material. However, the chemical structures of the tentatively identified ellagitannins were not determined, and studies on these constituents are also incomplete. Therefore, it is important to note although this is a preliminary study to provide a baseline of data for future investigations, a major limitation is that identified phyto-constituents were neither isolated, nor separately analyzed for their bioactivities. Moreover, the association between these compounds and antioxidant effect of pomegranate leaf is yet to be well understood. In this regard, it is necessary to further characterize the structure of these less-exploited phenolics (tannins) and their associated biological properties within pomegranate leaf. Hence, the results presented in our study confirm pomegranate leaf as a promising natural alternative in the development of antioxidant products, thereby assisting in the prevention and treatment of some diseases.
Conclusions
The level of different phenolic classes, antioxidant capacities and the phenolic profiles of seven medicinal and food plants were evaluated and correlated, including the leaves of sage, rosemary, olive, and pomegranate, as well as the leaves and young stems of rue, peppermint, and parsley. This study compared and demonstrated these plant extracts as valuable sources of bioactive compounds, likely for preparing novel functional products in various industries. High correlations of phenolic composition with antioxidant potential were investigated in our analysis. Different kinds of phenolic acids and flavonoids along with their derivatives were found widespread in the studied plant materials. Phenylethanoids especially oleuropein and its derivatives were characterized as the most abundant constituents of olive leaf extracts, probably contributing to its beneficial biological properties. While tannins particularly ellagitannins were supposed to be the main contributor to the features of pomegranate leaf. Interestingly, our results highlighted that the hydro-methanolic extracts of Punica granatum L. (pomegranate) leaves displayed the greatest levels of free radical scavenging capacity and ferric reducing antioxidant power, as well as the highest contents of total phenols, ortho -diphenols and tannins; a relatively high content of flavonoids was also found. Studies have increasingly evidenced the close association of tannins and less-studied compounds with antioxidant activity in medicinal and food plants 12 , 18 , 19 , 26 , 48 . Thus it is expected that richer phenolic types, namely tannins and phenolic glycosides, and their higher concentrations, are maintained in pomegranate leaves, making it possible to explore active ingredients and bioavailable products in the food-pharm, nutraceutical or cosmeceutical industries.
Moreover, only a limited number of researches have pointed out the comparison of biological activities and phenolic components of the tested plant organs, which belong to tree plants or shrub plants with large or small leaves. Many authors have stated the importance of vegetables, fruits, medicinal and aromatic plants in the current dietary patterns 2 , 3 , 4 , 5 , 29 , 30 , 50 . However, it doesn’t mean the agricultural and industrial waste like the tree leaves are useless for application. Extracts of olive leaves have attracted more attention recently, being reviewed as promising cheap, renewable and plenty source of bio-phenols for by-products. Some articles proved pomegranate leaf as a safe substrate due to its lower or inexistent toxicity 17 , 35 . In addition, ellagitannins as effective ingredients in teas are considered to be more abundant in the large-leaf tree than those from the small-leaf tree 61 , 62 . Therefore, as per olive leaf, research into finding new uses for by-products of pomegranate leaf may be proved as a strong argument for not only promoting human health but also improving bio-valorization and environment. However, samples of pomegranate leaves were not collected from different varieties or different seasons. Hence, studies on these issues would be of much interest in the future, in order to select the most promising matrix of the wasted bio-phenol materials.
Materials and methods
Chemicals and standards.
Compounds: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS ·+ ), (±)-6-hydroxy-2,5,7,8-tetramethylchromone-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhidrazyl radical (DPPH · ), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), sodium carbonate, sodium molybdate, potassium persulfate, and hydrochloric acid, all extra pure (> 99%) were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Reagents: ferric chloride, methanol, aluminum chloride, sodium nitrite, all extra pure (> 99%), and methyl cellulose (1500 centipoises viscosity at 2%) were acquired from Merck (Merck, Darmstadt, Germany). Sodium hydroxide, ammonium sulfate, Folin-Ciocalteu’s reagent and acetic acid, all extra pure (> 99%) were purchased from Panreac (Panreac Química S.L.U., Barcelona, Spain). Authentic standards of phenolic compounds used in the chromatographic analysis, including that protocatechuic acid (> 97%), p -hydroxybenzoic acid (> 99%), benzoic acid (> 99.5%) were obtained from Fluka (Fluka Chemika, Neu-Ulm, Switzerland), and caffeic acid (> 98%) was from Panreac (Panreac Química S.L.U., Barcelona, Spain). Standards: neochlorogenic acid (> 95%), chlorogenic acid (> 99%), vanillic acid (> 97%), syringic acid (≥ 99%), myricitin-3- O -glucoside (≥ 99%), p -coumaric acid (> 99%), rutin (quercetin-3-rutinoside) (≥ 94%), ellagic acid (≥ 95%), ferulic acid (> 99%), apigenin-7- O -glucoside (≥ 95%), rosmarinic acid (≥ 98%), luteolin (≥ 98%), quercetin (> 95%), trans -cinnamic acid (> 95%), and kaempferol (> 90%) were purchased from Chem-Lab (Chem-Lab N.V., Zedelgem, Belgium). Gallic acid (> 97.5%), tyrosol (> 98%), caftaric acid (≥ 97%), catechin (≥ 98%), gentisic acid (≥ 98%), epicatechin (≥ 98%), 4-hydrocinnamic acid (> 95%), luteolin-7- O -glucoside (≥ 98%), isorhamnetina-3- O -glucoside (> 95%), oleuropein (> 98%), resveratrol (≥ 99%), and trans -stilben (> 96%) were acquired from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Chromatography solvents were of RP-HPLC-DAD grade according to the analysis performed. Ultrapure water was obtained using a Water Purification System (Arioso Power, Human Corporation, Seoul, Korea).
Plant materials
From about one-hundred common medicinal and food plants reported in literature references, we have selected seven medicinal and food plants (Table S1 ) in this study according to following criteria: (1) higher phenolic content and antioxidant capacity, (2) lower or inexistent toxicity. Plant species were botanically authenticated by Prof. António Crespí (Department of Biology and Environment, University of Trás-os-Montes e Alto Douro, UTAD, Portugal) and Dr. João Rocha (Chemistry Centre-Vila Real, UTAD, Portugal). Samples of each species were hand-picked randomly from a pool of individual specimens (n > 10) that are naturally growing in the Botanical Garden of UTAD (Vila Real, Portugal), which belongs to the international network of botanical gardens. Sage, rosemary, rue, peppermint, and parsley are present in the Aromatic and Medicinal Plants collection; olive is present in the Mediterranean Calcareous collection; pomegranate is present in the Garden Fruits collection (more detailed information of each plant species can be checked at http://jb.utad.pt/ ). Thus, a mixture sample for each species was obtained and used for the subsequent analysis. The collected samples were immediately dried at 40 ℃ (Drying Cabinet, LEEC, Nottingham, UK) for 72 h, before being ground into a fine powder with a blender (MB 800, KINEMATICA AG, Malters, Switzerland), and hermetically stored in the dark, at room temperature (RT) until analysis. Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material have complied with relevant institutional, national, and international guidelines and legislation.
Preparation of plant phenolic extracts
The sample powder of each species was weighed and extracted in triplicate with 40 mg of dry weight (DW). The extraction was performed by agitating (30 min, 200 rpm, RT) the mixture of the powder and 1.5 mL of a hydro-methanolic solution (methanol:H 2 O, 70:30, v/v) in an orbital shaker (GFL 3005, GEMINI, Apeldoorn, Netherlands). Afterwards, the suspensions were centrifuged (10,000 rpm, 4 ℃) for 15 min (Sigma 2-16KL Refrigerated Centrifuges, Sigma Laborzentrifugen, Berlin, Germany). The supernatants were collected in a 5 mL volumetric flask, and the solid residues were then extracted twice via the same procedure. All the three supernatants from successive extractions were kept together and the final volume came to 5 mL with the above-mentioned extraction solvent.
Content of different phenolic classes
The content of total phenols, ortho -diphenols, and flavonoids was determined by colorimetric and spectrophotometric approaches according to the literature 63 . The content of tannins was evaluated by the methyl cellulose (MC) methodology previously reported by Dambergs et al. 64 .
For the determination of total phenol content, 20 μL of diluted sample, 100 μL of diluted Folin-Ciocalteu reagent (90%, v/v), and 80 μL aqueous sodium carbonate (7.5%, w/v) were mixed in sequence. The mixture was incubated for 30 min at 42 ℃ in the dark and measured at 750 nm, using gallic acid as standard. Results were expressed in milligrams of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW).
For the assessment of ortho -diphenols content, 40 μL of sodium molybdate solution (5%, w/v) prepared with hydro-methanol (50%, v/v) was added to 160 μL of diluted extract. The mixture was stood for 15 min at RT, protected from light, before the absorbance at 375 nm was read. The content was quantified using gallic acid as standard. Results were defined in mg GAE g −1 DW.
For the quantification of total flavonoids content, 24 μL of diluted extract and 28 μL of sodium nitrite (5%, w/v) were mixed. After 5 min at RT, 28 μL of a 10% (w/v) aluminum chloride solution was added in the mixture and reacted for 6 min. Afterwards, 120 μL of sodium hydroxide (1 M) was added and the final mixture was read at 520 nm after agitation for 30 s in a microplate reader. The results were expressed in milligrams of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW).
The above-mentioned assays were undertaken with a microplate reader (Multiskan FC Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland) in 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) with a final volume of 200 µL.
The content of tannins was evaluated both in treatment and control groups simultaneously, by adding 600 μL of methyl cellulose (MC) solution (treatment) or water (control) to 200 μL of sample in a 2 mL Eppendorf. The mixture was stirred manually for 2–3 min at RT. Four hundred μL of saturated ammonium sulfate and 800 μL of water were added successively both in the treatment and control groups until 2 mL of total volume was reached. The final mixture was vortexed and kept for 10 min. After centrifugation (10,000 rpm, 16 ℃, 5 min), the absorbance was read at 280 nm, by using a conventional spectrophotometer (Helios Gamma UV Spectrophotometer, Thermo Electron Corporation, Warwickshire, UK). The absorbance of tannins was obtained by subtracting the treatment absorbance from the value registered from the control, using epicatechin as standard. The results were described in milligrams of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW).
Evaluation of in vitro antioxidant activity
The antioxidant activity of sample extracts was determined by ABTS, DPPH and FRAP (ferric reducing antioxidant power) spectrophotometric methods, reported by Mena et al. 65 , with some modifications.
The ABTS + radicals were produced by mixing 5 mL of ABTS stock solution (7.0 mM) with 88 μL of potassium persulfate (148 mM), and diluted to a working solution with sodium acetate buffer (20 mM, pH 4.5), showing an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 188 μL of ABTS working solution and 12 μL of sample dilutions (water used as blank) were mixed and reacted for 30 min at RT, and then the absorbance was read at 734 nm.
The DPPH radicals (8.87 mM) were formed with methanol (99.9%) and diluted in a working solution with hydro-methanol (70%, v/v), achieving an absorbance of 1000 at 520 nm. A mixture of 190 μL of DPPH working solution and 10 μL of sample dilutions (70% hydro-methanol used as blank) was incubated for 15 min at RT, reading the absorbance at 520 nm.
The FRAP working solution was prepared by mixing 10-volume acetate buffer (300 mM, pH 3.6), 1-volume TPTZ (10 mM dissolved in hydrochloric acid), and 1-volume ferric chloride (20 mM in water). The mixture was maintained at 37 ℃ for 10 min before use. The reaction of FRAP working solution (180 μL) and sample dilutions (20 μL) was kept at 37 ℃ for 30 min and the absorbance read at 593 nm.
The three antioxidant assays were adapted to microscale using 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) and microplate readers (Multiskan GO Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland), using Trolox as standard. All the results were expressed in millimoles of Trolox per gram of plant dry weight (mM Trolox g −1 DW).
Reverse phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) system (Thermo Finnigan, San Diego, CA, USA) was carried out to determine the (poly)phenolic profile of each plant extract, as previously described 63 . The analysis equipment is composed of three parts, including LC pump (Surveyor), autosampler (Surveyor), and PDA detector (Surveyor). Sample extracts, in triplicate, and 31 pure standard compounds (all in HPLC grade), including 17 phenolic acids, 10 flavonoids, 2 phenylethanoids and 2 stilbenoids, were prepared and filtered through 0.45 μm PVDF filters (Millex-HV Syringe Filter Unit, Merck Millipore, Bedford, MA, USA) and injected into a C18 column (250 × 4.6 mm, 5 μm particle size; ACE, Aberdeen, Scotland), using a mobile phase composed of water/formic acid (99.9:0.1, v/v) (solvent A) and acetonitrile/formic acid (99.9:0.1, v/v) (solvent B). The linear gradient program (t in min and %B) was: t = 0–0%; t = 5–0%; t = 20–20%; t = 35–50%; t = 40–100%; t = 45–0%; and t = 65–0%. The injection volume was 20 μL and the flow rate was kept at 1.0 mL min −1 . UV/Vis detection was recorded from 200 to 600 nm range. Peaks were monitored at 280 and 330 nm, and identified by congruent retention time compared with standards. Data acquisition, peak integration and analysis were performed using Chromeleon software (Version 7.1; Thermo Scientific, Dionex, USA). The three extracts of each medicinal plant were chromatographed and results were expressed in milligram per liter of sample extracts (mg L −1 ).
Data and statistical analysis
All the measurements of phenolic phytochemicals and antioxidant activity of the plant extracts were conducted in triplicate. The results of phenolic content and antioxidant activity are presented as mean ± standard deviation (SD). Concentrations of individual identified phenolic compounds are presented as mean (n = 3) with the determination of the Least Significant Difference (LSD) for a p value < 0.05. The obtained data were subjected to analysis of variance (ANOVA) and a multiple range test (Tukey’s test) with IBM SPSS statistics 21.0 software (SPSS Inc., Chicago, USA). Pearson ( r ) analysis was carried out to establish correlations between phenolic chemical classes and antioxidant activity.
Li, Q. et al. Cholinesterase, β -amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crops Prod. 108 , 512–519. https://doi.org/10.1016/j.indcrop.2017.07.001 (2017).
Article CAS Google Scholar
Nollet, L. M. & Gutierrez-Uribe, J. A. Phenolic Compounds in Food: Characterization and Analysis 1st edn, 167 (CRC Press, 2018).
Book Google Scholar
Uritu, C. M. et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018 , 7801543. https://doi.org/10.1155/2018/7801543 (2018).
Article PubMed PubMed Central Google Scholar
Etkin, N. L. Plants and Indigenous Medicine and Diet: Biobehavioral Approaches eBook, 336 (Taylor & Francis, 2019).
Watson, R. R. & Preedy, V. R. Fruits, Vegetables, and Herbs: Bioactive Foods in Health Promotion 1st edn. (Elsevier Academic Press, 2016).
Google Scholar
Alavi, M. S., Fanoudi, S., Ghasemzadeh Rahbardar, M., Mehri, S. & Hosseinzadeh, H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother. Res. https://doi.org/10.1002/ptr.6894 (2020).
Article PubMed Google Scholar
Ghorbani, A. & Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 7 , 433–440. https://doi.org/10.1016/j.jtcme.2016.12.014 (2017).
Mahendran, G. & Rahman, L.-U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint ( Mentha × piperita L.)—a review. Phytother. Res. 34 , 2088–2139. https://doi.org/10.1002/ptr.6664 (2020).
Shamal Badhusha, P. A. et al. Traditional uses, phytochemistry and ethanopharmacology of Ruta graveolens Linn: A review. Int. J. Pharm. Drug Anal. 20 , 1–4 (2020).
Colucci-D’Amato, L. & Cimaglia, G. Ruta graveolens as a potential source of neuroactive compounds to promote and restore neural functions. J. Tradit. Complement. Med. 10 , 309–314. https://doi.org/10.1016/j.jtcme.2020.05.002 (2020).
Şahin, S. & Bilgin, M. Olive tree ( Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 98 , 1271–1279. https://doi.org/10.1002/jsfa.8619 (2018).
Article CAS PubMed Google Scholar
Salama, Z. A. et al. In-vitro antioxidant, antimicrobial and anticancer activities of banana leaves ( Musa acuminata ) and olive leaves ( Olea europaea L.) as by-products. Res. J. Pharm. Technol. 13 , 687–696. https://doi.org/10.5958/0974-360X.2020.00132.8 (2020).
Article Google Scholar
Farzaei, M. H., Abbasabadi, Z., Ardekani, M. R. S., Rahimi, R. & Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 33 , 815–826. https://doi.org/10.1016/S0254-6272(14)60018-2 (2013).
Cefali, L. C. et al. Evaluation of in vitro solar protection factor (SPF), antioxidant activity, and cell viability of mixed vegetable extracts from Dirmophandra mollis Benth, Ginkgo biloba L., Ruta graveolens L., and Vitis vinífera L.. Plants 8 , 453. https://doi.org/10.3390/plants8110453 (2019).
Article CAS PubMed Central Google Scholar
Mara de Menezes Epifanio, N. et al. Chemical characterization and in vivo antioxidant activity of parsley ( Petroselinum crispum ) aqueous extract. Food Funct. 11 , 5346–5356. https://doi.org/10.1039/D0FO00484G (2020).
Vučić, V., Grabež, M., Trchounian, A. & Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 25 , 1817–1827. https://doi.org/10.2174/1381612825666190708183941 (2019).
Viswanatha, G. L., Venkataranganna, M. V., Prasad, N. B. L. & Ashok, G. Evaluation of anti-epileptic activity of leaf extracts of Punica granatum on experimental models of epilepsy in mice. J. Intercult. Ethnopharmacol. 5 , 415–421. https://doi.org/10.5455/jice.20160904102857 (2016).
Article CAS PubMed PubMed Central Google Scholar
Yeddes, W., Chalghoum, A., Aidi-Wannes, W., Ksouri, R. & Saidani Tounsi, M. Effect of bioclimatic area and season on phenolics and antioxidant activities of rosemary ( Rosmarinus officinalis L.) leaves. J. Essential Oil Res. 31 , 432–443. https://doi.org/10.1080/10412905.2019.1577305 (2019).
Christova-Bagdassarian, V. L., Bagdassarian, K. S., Atanassova, M. S. & Ahmad, M. A. Comparative analysis of total phenolic and total flavonoid contents, rutin, tannins and antioxidant capacity in Apiaceae and Lamiaceae families. Indian Hortic. J. 4 , 131–140 (2014). https://www.academia.edu/15503276/Comparative_Analysis_of_Total_Phenolic_and_Total_Flavonoid_Contents_Rutin_Tannins_and_Antioxidant_Capacity_in_Apiaceae_and_Lamiaceae_families?source=swp_share
Asgharian, S. et al. Ruta graveolens and rutin, as its major compound: Investigating their effect on spatial memory and passive avoidance memory in rats. Pharm. Biol. 58 , 447–453. https://doi.org/10.1080/13880209.2020.1762669 (2020).
Aissani, N., Albouchi, F. & Sebai, H. Anticancer effect in human glioblastoma and antioxidant activity of Petroselinum crispum L. methanol extract. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1842894 (2020).
Farnad, N., Heidari, R. & Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint ( Mentha piperita ). J. Food Meas. Charact. 8 , 113–121. https://doi.org/10.1007/s11694-014-9171-x (2014).
Roby, M. H. H., Sarhan, M. A., Selim, K.A.-H. & Khalel, K. I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme ( Thymus vulgaris L.), sage ( Salvia officinalis L.), and marjoram ( Origanum majorana L.) extracts. Ind. Crops Prod. 43 , 827–831. https://doi.org/10.1016/j.indcrop.2012.08.029 (2013).
Dent, M., Dragović-Uzelac, V., Penić, M., Bosiljkov, T. & Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage ( Salvia officinalis L.) extracts. Food Technol. Biotech. 51 , 84–91 (2013).
CAS Google Scholar
Mulinacci, N. et al. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 85 , 167–176. https://doi.org/10.1016/j.talanta.2011.03.050 (2011).
Ramkissoon, J. S., Mahomoodally, M. F., Ahmed, N. & Subratty, A. H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 6 , 561–569. https://doi.org/10.1016/S1995-7645(13)60097-8 (2013).
Mestry, S. N., Dhodi, J. B., Kumbhar, S. B. & Juvekar, A. R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 7 , 273–280. https://doi.org/10.1016/j.jtcme.2016.06.008 (2017).
Uysal, S., Zengin, G., Aktumsek, A. & Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 22 , 518–532. https://doi.org/10.1016/j.jff.2016.02.006 (2016).
Kaewnarin, K., Niamsup, H., Shank, L. & Rakariyatham, N. Antioxidant and antiglycation activities of some edible and medicinal plants. Chiang Mai J. Sci. 41 , 105–116 (2014).
Cai, Y., Luo, Q., Sun, M. & Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74 , 2157–2184. https://doi.org/10.1016/j.lfs.2003.09.047 (2004).
Elfalleh, W. et al. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 6 , 4724–4730 (2012).
Fellah, B. et al. Untargeted metabolomics reveals changes in phenolic profile following in vitro large intestine fermentation of non-edible parts of Punica granatum L.. Food Res. Int. 128 , 108807. https://doi.org/10.1016/j.foodres.2019.108807 (2020).
Pinheiro, A. J. M. C. R. et al. Punica granatum L. leaf extract attenuates lung inflammation in mice with acute lung injury. J. Immunol. Res. 2018 , 1–11. https://doi.org/10.1155/2018/6879183 (2018).
Ulewicz-Magulska, B. & Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Food Hum. Nutr. 74 , 61–67. https://doi.org/10.1007/s11130-018-0699-5 (2019).
Ankita, P., Deepti, B. & Nilam, M. Flavonoid rich fraction of Punica granatum improves early diabetic nephropathy by ameliorating proteinuria and disturbed glucose homeostasis in experimental animals. Pharm. Biol. 53 , 61–71. https://doi.org/10.3109/13880209.2014.910533 (2015).
Generalić, I. et al. Influence of the phenophase on the phenolic profile and antioxidant properties of Dalmatian sage. Food Chem. 127 , 427–433. https://doi.org/10.1016/j.foodchem.2011.01.013 (2011).
Salwe, K. J. & Sachdev, D. Evaluation of antinociceptive and anti-inflammatory effect of the hydroalcoholic extracts of leaves and fruit peel of P. granatum in experimental animals. Asian J. Pharm. Clin. Res. 7 , 137–141 (2014).
AlFadel, F., Al Laham, S. & Alkhatib, R. The anti-bacterial activity of various parts of Punica granatum on antibiotics resistance Escherichia coli . Seeds 21 , 76 (2014).
Gheith, I. & El-Mahmoudy, A. Potent anti-oxidant and anti-inflammatory potentials of Punica granatum leaf and flower hydromethanolic extracts in vitro. Biosci. J. https://doi.org/10.14393/BJ-v33n2-33736 (2017).
Pararin, S., Rouhi, L. & Ghasemi Pirbalouti, A. The beneficial effect of hydro-alcoholic extract of Punica granatum L. leaves and flower on ethylene glycol-induced kidney calculi in RATS. J. Herb. Drugs (Int. J. Med. Herbs) 7 , 59–64 (2016).
Kiraz, Y., Neergheen-Bhujun, V. S., Rummun, N. & Baran, Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 37 , 1803–1815. https://doi.org/10.1007/s13277-015-3962-5 (2016).
Rummun, N., Somanah, J., Ramsaha, S., Bahorun, T. & Neergheen-Bhujun, V. S. Bioactivity of nonedible parts of Punica granatum L.: A potential source of functional ingredients. Int. J. Food Sci. 2013 , 602312. https://doi.org/10.1155/2013/602312 (2013).
Kapp, K. et al. Commercial peppermint ( Mentha × piperita L) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 53 , 758–766. https://doi.org/10.1016/j.foodres.2013.02.015 (2013).
Meziane-Assami, D., Tomao, V., Ruiz, K., Meklati, B. Y. & Chemat, F. Geographical differentiation of rosemary based on GC/MS and fast HPLC analyses. Food Anal. Method 6 , 282–288. https://doi.org/10.1007/s12161-012-9430-6 (2013).
Khaleel, I. R., Kadhim, M. I. & Subhi, J. H. Effect of some biotic and abiotic elicitors on phenolic acids and diterpenes production from rosemary ( Rosmarinus officinalis L.) leaf and callus analyzed by high performance liquid chromatography (Hplc). Al-Nahrain J. Sci. 14 , 20 (2018).
Melnyk, M., Vodoslavskyi, V. & Obodianskyi, M. Research of phenolic compounds of ruta graveolens l and stellaria media (l.) vill. Asian J. Pharm. Clin. Res. 11 , 152–156 (2018).
Figueroa-Pérez, M. G. et al. Diabetic nephropathy is ameliorated with peppermint ( Mentha piperita ) infusions prepared from salicylic acid-elicited plants. J. Funct. Foods 43 , 55–61. https://doi.org/10.1016/j.jff.2018.01.029 (2018).
Dekanski, D. et al. Phytochemical analysis and gastroprotective activity of an olive leaf extract. J. Serb. Chem. Soc. 74 , 367–377. https://doi.org/10.2298/JSC0904367D (2009).
Makowska-Wąs, J. et al. Identification of predominant phytochemical compounds and cytotoxic activity of wild olive leaves ( Olea europaea L. ssp. sylvestris) harvested in South Portugal. Chem. Biodiv. 14 , e1600331. https://doi.org/10.1002/cbdv.201600331 (2017).
Skotti, E. et al. Biological activity of selected Greek medicinal and aromatic plants extracts on Alternaria alternata. Emirates J. Food Agric. 28 , 796–804. https://doi.org/10.9755/ejfa.2016-06-618 (2016).
Arruda, M. O. et al. The hydroalcoholic extract obtained from Mentha piperita L. leaves attenuates oxidative stress and improves survival in lipopolysaccharide-treated macrophages. J. Immunol. Res. 2017 , 2078794. https://doi.org/10.1155/2017/2078794 (2017).
Luthria, D. L., Mukhopadhyay, S. & Kwansa, A. L. A systematic approach for extraction of phenolic compounds using parsley ( Petroselinum crispum ) flakes as a model substrate. J. Sci. Food Agric. 86 , 1350–1358. https://doi.org/10.1002/jsfa.2521 (2006).
Hozayen, W. G., El-Desouky, M. A., Soliman, H. A., Ahmed, R. R. & Khaliefa, A. K. Antiosteoporotic effect of Petroselinum crispum , Ocimum basilicum and Cichorium intybus L. in glucocorticoid-induced osteoporosis in rats. Bmc. Complement. Altern. Med. 16 , 165. https://doi.org/10.1186/s12906-016-1140-y (2016).
Marques, L. C. et al. Anti-inflammatory effects of a pomegranate leaf extract in LPS-induced peritonitis. Planta Med. 82 , 1463–1467. https://doi.org/10.1055/s-0042-108856 (2016).
Swilam, N. & Nematallah, K. A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 10 , 14851. https://doi.org/10.1038/s41598-020-71847-5 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Akkawi, M., Abu-Lafi, S. & Abu-Remeleh, Q. Phytochemical screening of Pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm. Pharmacol. Int. J. 7 , 193–200 (2019).
Pinheiro, A. C. et al. Galloyl-hexahydroxydiphenoyl (HHDP)-glucose isolated from Punica granatum L. leaves protects against lipopolysaccharide (LPS)-induced acute lung injury in BALB/c mice. Front. Immunol. 10 , 1978. https://doi.org/10.3389/fimmu.2019.01978 (2019).
Lakshminarayanashastry Viswanatha, G., Venkatanarasappa Venkataranganna, M. & Lingeswara Prasad, N. B. Methanolic leaf extract of Punica granatum attenuates ischemia-reperfusion brain injury in Wistar rats: Potential antioxidant and anti-inflammatory mechanisms. Iran. J. Basic Med. Sci. 22 , 187–196. https://doi.org/10.22038/ijbms.2018.30660.7389 (2019).
Chen, J. et al. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 10 , 2611. https://doi.org/10.1038/s41598-020-59451-z (2020).
Xue, Z. & Yang, B. Phenylethanoid glycosides: Research advances in their phytochemistry, pharmacological activity and pharmacokinetics. Molecules (Basel, Switzerland) 21 , 991. https://doi.org/10.3390/molecules21080991 (2016).
Fraga-Corral, M. et al. Technological application of tannin-based extracts. Molecules 25 , 614. https://doi.org/10.3390/molecules25030614 (2020).
Yang, X. & Tomás-Barberán, F. A. Tea is a significant dietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 67 , 5394–5404. https://doi.org/10.1021/acs.jafc.8b05010 (2019).
Gouvinhas, I. et al. Monitoring the antioxidant and antimicrobial power of grape ( Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 126 , 83–91. https://doi.org/10.1016/j.indcrop.2018.10.006 (2018).
Dambergs, R. G., Mercurio, M. D., Kassara, S., Cozzolino, D. & Smith, P. A. Rapid measurement of methyl cellulose precipitable tannins using ultraviolet spectroscopy with chemometrics: Application to red wine and inter-laboratory calibration transfer. Appl. Spectrosc. 66 , 656–664. https://doi.org/10.1366/11-06516 (2012).
Article ADS CAS PubMed Google Scholar
Mena, P. et al. Phytochemical characterisation for industrial use of pomegranate ( Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 91 , 1893–1906. https://doi.org/10.1002/jsfa.4411 (2011).
Download references
Acknowledgements
The experiments were approved by the FCT-Portuguese Foundation for Science and Technology (PD/BD/135333/2017), under the Doctoral Programme “Agricultural Production Chains-from fork to farm” (PD/00122/2012).
This research was funded by the FCT (Portuguese Foundation for Science and Technology) Grant number UIDB/04033/2020.
Author information
Authors and affiliations.
Centre for the Research and Technology of Agro-Environmental and Biological Sciences, CITAB, University de Trás-os-Montes e Alto Douro, UTAD, 5000-801, Vila Real, Portugal
Manyou Yu, Irene Gouvinhas & Ana I. R. N. A. Barros
Chemistry Centre-Vila Real, CQ-VR, UTAD, 5000-801, Vila Real, Portugal
Department of Chemistry, School of Life Sciences and Environment, UTAD, Quinta de Prados, 5001-801, Vila Real, Portugal
Ana I. R. N. A. Barros
You can also search for this author in PubMed Google Scholar
Contributions
M.Y. carried out data analysis, wrote the manuscript, and participated in all experimental measurements. I.G. developed and performed the chromatographic analysis. J.R. supervised botanical identification and sample collection. A.I.R.N.A.B. conceived all experiments, performed theoretical calculations, and supervised data analysis and interpretation. All authors reviewed the manuscript and participated in editing the manuscript.
Corresponding authors
Correspondence to Manyou Yu or Ana I. R. N. A. Barros .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yu, M., Gouvinhas, I., Rocha, J. et al. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep 11 , 10041 (2021). https://doi.org/10.1038/s41598-021-89437-4
Download citation
Received : 04 January 2021
Accepted : 27 April 2021
Published : 11 May 2021
DOI : https://doi.org/10.1038/s41598-021-89437-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Fatty acid and nutrient profiles, diosgenin and trigonelline contents, mineral composition, and antioxidant activity of the seed of some iranian trigonella l. species.
- Ziba Bakhtiar
- Mohammadreza Hassandokht
- Mohammad Hossein Mirjalili
BMC Plant Biology (2024)
Insight into antioxidant-like activity and computational exploration of identified bioactive compounds in Talinum triangulare (Jacq.) aqueous extract as potential cholinesterase inhibitors
- Olakunle Bamikole Afolabi
- Oluwaseun Ruth Olasehinde
- Oghenerobor Benjamin Akpor
BMC Complementary Medicine and Therapies (2024)
Integrated metabolomic and transcriptomic dynamic profiles of endopleura coloration during fruit maturation in three walnut cultivars
- Hengzhao Liu
- Huijuan Zhou

Biosynthesis of CuO nanoparticle using leaf extracts of Ocimum lamiifolium Hochst. ex Benth and Withana somnifera (L) Dunal for antibacterial activity
- Sebesibe Mulunehe Mengesha
- Gedif Meseret Abebe
- Tesfaye Haile Habtemariam
Scientific Reports (2024)
GC–MS based metabolomic profiling of Aporosa cardiosperma (Gaertn.) Merr. leaf extracts and evaluating its therapeutic potential
- Ubais Abdul
- Dinesh Babu Manikandan
- Thirumurugan Ramasamy
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Antioxidant potential of medicinal plants
- Review Article
- Published: 21 June 2022
- Volume 26 , pages 13–26, ( 2023 )
Cite this article

- S. S. Sravanthi Pammi ORCID: orcid.org/0000-0003-0984-8211 1 ,
- Bastipati Suresh 2 &
- Archana Giri 2
1341 Accesses
14 Citations
Explore all metrics
Medicinal plants are used for health care either directly or indirectly as they are rich source of several ingredients. Ethno-medical traditions prove that they have a great therapeutic value and are important bioresources. Phytochemicals are naturally occurring compounds having immense antioxidant potential and are of great interest in securing health benefits of consumers. Each portion of the plant has its own medicinal properties possessing different types of secondary metabolites which plays important role in treatment of different types of diseases and for manufacturing of drugs. The phytopharmaceutical preparations should be safely assessed and well established before their usage. In spite of medicinal uses, phytochemicals have also been used in cosmetics, fragrance and as food supplements. Global research is recently focusing on search of new medicines or active compounds with proven significant scientific output. This review focusses on the metabolic fingerprint and biological properties of various plants which play a major role in antioxidant activity along with their mechanisms of action.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price excludes VAT (USA) Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Antioxidant Potential of African Medicinal Plants
Plants of indian traditional medicine with antioxidant activity.

Medicinal Plants: A Rich Source of Bioactive Molecules Used in Drug Development
Ahmed M, Phul AR, Bibi G, Mazhar K, Ur-Rehman T, Zia M, Mirza B (2016) Antioxidant, anticancer and antibacterial potential of Zakhm-e-hayat rhizomes crude extract and fractions. Pak J Pharmaceut Sci 29(3):895–902
CAS Google Scholar
Ahmeda A, Amzad Hossain M, Ismail Z (2009) Antioxidant properties of the isolated flavonoids from the medicinal plant Phyllanthus niruri . Asian J Food and Agro-Ind 2(03):373–381
Google Scholar
Akporowhe S, Onyesom I (2016) Phyllanthus amarus augments the serum antioxidant capacity and invigorates the blood in experimental mice. Biosci Biotechnol Res Commun 9(1):15–18
Article Google Scholar
Anil Kumar M, Pammi SSS, Archana G (2017) Establishment of suspension cultures and production of isoflavones from red clover ( Trifolium pratense L.). Int J Appl Biotechnol Biochem (IJABB) 1(1):35–42
Annadurai G, Masilla BR, Jothiramshekar S, Palanisami E, Puthiyapurayil S, Parida AK (2012) Antimicrobial, antioxidant, anticancer activities of Syzygium caryophyllatum (L.) Alston. Int J Green Pharm 6(4):285–288
Anwar H, Hussain G, Mustafa I (2018) Antioxidants from natural sources. In: Shalaby E, Azzam GM (eds) Antioxidants in foods and its applications. IntechOpen, London. https://doi.org/10.5772/intechopen.75961
Chapter Google Scholar
Arias A, Feijoo G, Moreira MT (2022) Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov Food Sci Emerg Technol 77(2022):102974. https://doi.org/10.1016/j.ifset.2022.102974
Asraoui F, Kounnoun A, Cadi HE, Cacciola F, Majdoub YO, Alibrando F, Mandolfino F, Dugo P, Mondello L, Louajri A (2021) Phytochemical investigation and antioxidant activity of Globularia alypum L. Molecules 26(3):759. https://doi.org/10.3390/molecules26030759
Article CAS Google Scholar
Atanasov AG, Zotchev SB, Dirsch VM (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 20:200–216. https://doi.org/10.1038/s41573-020-00114-z
Bailey BA, Strema MD, Bae H, Antunezde Mayolo G, Guiltinan MJ (2005) Gene expression in leaves of Theobroma cacao in response to mechanical wounding, ethylene, and/or methyl jasmonate. Plant Sci 168:1247–1258
Bardaweel SK, Khaled AT, Mohammad MH (2014) Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement Altern Med 14:297
Bari MW, Islam A, Islam MM, Sultana MJ, Afroz R, Khan MM, Parul SS, Swaraz AM, Hossain MI, Islam MA (2021) Determination of in vitro antioxidant activity and in vivo antineoplastic effects against Ehrlich ascites carcinoma of methanolic extract of Sphagneticola calendulacea (L.) Pruski. Heliyon 7(6):228. https://doi.org/10.1016/j.heliyon.2021.e07228
Bhakya S, Muthukrishnan S, Sukumaran M, Grijalva M, Cumbal L, Franklin Benjamin JH, KumarT S, Rao MV (2016) Antimicrobial, antioxidant and anticancer activity of biogenic silver nanoparticles – an experimental report. R Soc Chem Adv 6:81436–81446
Bharat G, Kothari IL (2011) Antioxidant and antimicrobial activity of in vivo and in vitro grown plants of Phyllanthus niruri . Int J Pharm Biol Sci 2(2):78–89
Bhatt ID, Rawat S, Rawal RS (2013) Antioxidants in medicinal plants. Biotechnol Med Plants 13:295–326
Braga TV, das Dores RG, Ramos CS, Evangelista FC, da Silva Tinoco LM, de Pilla Varotti F, das Graças Carvalho M, de Paula-Sabino A (2014) Antioxidant, antibacterial and antitumor activity of ethanolic extract of the Psidium guajava leaves. Am J Plant Sci 5(23):3492
Brunet CJ, Gordana C, Sonja D, Vesna T, Gordana B, Anamarija M, Sinisa M, Dragoljub C, Vladimir C (2008) Radical scavenging, antibacterial, and antiproliferative activities of Melissa officinalis L. extracts. J Med Food 11(1):133–143
da Sarmento NC, Apilak W, Ratchanok P, Supaluk P, Somsak R, Virapong P (2015) Antimicrobial, antioxidant and anticancer activities of Strychnos lucida R.Br. Afr J Tradit Complement Altern Med 12(4):122–127
Decros G, Baldet P, Beauvoit B, Stevens R, Flandin A, Colombié S, Gibon Y, Pétriacq P (2019) Get the balance right: ROS homeostasis and redox signalling in fruit. Front Plant Sci 10:1091
Farooq S, Ngaini Z (2021) Natural and synthetic drugs as potential treatment for coronavirus disease 2019 (COVID-2019). Chem Afr 4:1–13. https://doi.org/10.1007/s42250-020-00203-x
Fitzgerald M, Heinrich M, Booker A (2020) Medicinal plant analysis: a historical and regional discussion of emergent complex techniques. Front Pharmacol 10:1480. https://doi.org/10.3389/fphar.2019.01480
Gill SS, Anjum NA, Gill R, Yadav S, Hasanuzzaman M, Fujita M, Mishra P, Sabat SC, Tuteja N (2015) Superoxide dismutase: mentor of abiotic stress tolerance in crop plants. Environ Sci Pollut Res 22:10375–10394
Gorai D, Jash SK, Singh RK, Gangopadhyay A (2014) Chemical and pharmacological aspects of Limnophila aromatica (Scrophulariaceae): an overview. Am J Phytomed Clin Ther 2(3):348–356
Gul MZ, Bhakshu LM, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA (2011) Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement Altern Med 11:64
Gupta VK, Sharma SK (2006) Plants as natural antioxidants. Natl Prod Radiance 5(4):326–334
Haleem MA, Barton KL, Borges G, Crozier A, Anderson AS (2008) Increasing antioxidant intake from fruits and vegetables: practical strategies for the Scottish population. J Hum Nutr Diet 21(6):539–546
Hasanuzzaman M, Bhuyan M, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384
Hasanuzzaman M, Bhuyan M, Zulfiqar F, Raza A, Mohsin SM, Mahmud J, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Hole PS, Darley RL, Tonks A (2011) Do reactive oxygen species play a role in myeloid leukemias? Blood 117(22):5816–5826
Indrajith A, Ravindran KC (2009) Antioxidant potential of Indian medicinal plant Phyllanthus amarus under supplementary UV-B radiation. Recent Res Sci Technol 1(1):034–042
Inoue M, Hayashi S, Lyle E (2019) Role of medicinal and aromatic plants: past, present and future. In: Shagufta P, Areej A-T (eds) Pharmacognosy-medicinal plants. IntechOpen, London. https://doi.org/10.5772/intechopen.82497
Ismael J, Dessalegn E, Fereja WM (2021) In vitro antioxidant and antibacterial activity of leaf extracts of Measa lanceolate . Int J Food Prop 24(1):702–712. https://doi.org/10.1080/10942912.2021.1917608
Kabera JN, Semana E, Mussa AR, He X (2014) Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol 2:377–392
Kamagaté M, Koffi C, Kouamé NM, Akoubet A, Yao NA, Die-Kakou HM (2014) Ethnobotany, phytochemistry, pharmacology and toxicology profiles of Cassia siamea Lam. J Phytopharmacol 3(1):57–76
Karuna R, Reddy SS, Baskar R, Saralakumari D (2009) Antioxidant potential of aqueous extract of Phyllanthus amarus in rats. Indian J Pharmacol 41(2):64–67
Karuna R, Bharathi VG, Reddy SS, Ramesh B, Saralakumari D (2011) Protective effects of Phyllanthus amarus aqueous extract against renal oxidative stress in Streptozotocin-induced diabetic rats. Indian J Pharmacol 43(4):414–418
Kasote DM, Hegde MV, Katyare SS (2013) Mitochondrial dysfunction in psychiatric and neurological diseases: cause(s), consequence(s) and implications of antioxidant therapy. BioFactors 39(4):392–406
Kasote DM et al (2015) Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 11(8):982–991
Katiyar S, Patidar D, Gupta S, Singh RK, Singh P (2013) Some indian traditional medicinal plants with antioxidant activity: a review. Int J Innov Res Sci Eng Technol 2(12):7303–7314
Kennedy DO, Wightman EL (2011) Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr J 2:32–50
Krishnanada KI, Deshmukh AG, Padole DA, Dudhare MS, Moharil MP, Khelurkar VC (2017) Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J Pharmacogn Phytochem 6(1):32–36
Kumar N (2014) Plant profile, phytochemistry and pharmacology of Avartani ( Helicteres isora Linn.): a review. Asian Pac J Trop Biomed 4(1):S22–S26
Kurek M, Benaida-Debbache N, Elez Garofulic I, Galic K, Avallone S, Voilley A, Wache Y (2022) Antioxidants and bioactive compounds in food: critical review of issues and prospects. Antioxidants (basel) 11(4):742. https://doi.org/10.3390/antiox11040742
Lee SW, Wee W, Julius FJ, Siong Y, Syamsumir DF (2011a) Characterization of antioxidant, antimicrobial, anticancer property and chemical composition of Ficus deltoidea jack leaf extract. J Biol Active Prod Nat 1(1):1–6
Lee SW, Wendy W, Julius YFS, Desy FS (2011b) Characterization of antimicrobial, antioxidant, anticancer property and chemical composition of Michelia champaca seed and flower extracts. Stamford J Pharmaceut Sci 4(1):19–24
Lee SW, Wee W, Julius FS, Fitrya SD (2011c) Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med Iran 49(10):670–674
Lee J, Park G, Chang YH (2019) Nutraceuticals and antioxidant properties of Lonicera japonica Thunb. as affected by heating time. Int J Food Prop 22:630–645. https://doi.org/10.1080/10942912.2019.1599389
Li X, Xiaoting W, Huang L (2009) Correlation between antioxidant activities and phenolic contents of Radix Angelicae sinensis (Danggui). Molecules 14(12):5349–5361
Ling-Tong Q, Wang S-C, Zhang J (2015) Chemical constituents from Polygonatum odoratum . Biochem Syst Ecol 58:281–284
Logesh R, Dhanabal SP, Duraiswamy B, Chaitanya MVNL (2017) Medicinal plants diversity and their folklore uses by the tribes of Nilgiri Hills, Tamil Nadu, India. Int J Pharmacogn Chin Med 1(3):00
Lu J-M, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14(4):840–860
Madhuvanthi C, Santhosh Kumar K, Antony Ceasar S, Valivittan K, Srinivasan K, Tamilselvi A (2014) Antibacterial, antioxidant and antiproliferative activities of solvent extracts of Tiliacora acuminata . Int J Pharm Pharm Sci 6(9):398–403
Manyou Y, Irene G, Joao R, Ana IRNAB (2021) Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep 11:10041. https://doi.org/10.1038/s41598-021-89437-4
Masota NE, Vogg G, Heller E, Holzgrabe U (2020) Comparison of extraction efficiency and selectivity between low-temperature pressurized microwave-assisted extraction and prolonged maceration. Arch Pharmacal Res 353:2000147. https://doi.org/10.1002/ardp.202000147
Md. Habib R, Md. Mominur R, Raquibul HSM, Mohammed AS, Sohel R (2011) Antibacterial, cytotoxic and antioxidant potential of methanolic extract of Phyllanthus acidus . Int J Drug Dev Res 3(2):154–161
Meena J, Sharma RA, Rolania R (2017) A review on phytochemical and pharmacological properties of Phyllanthus amarus schum. and thonn. Int J Pharmaceut Sci Res 9(4):1377–1386
Mothana RA, Lindequist U, Gruenert R, Bednarski PJ (2009) Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement Altern Med 9:7
Na thalie SA, Karin K, Kristin E (2014). My life, my health, my community. Nutr Fit Workshop
Nanasombat S, Teckchuen N (2009) Antimicrobial, antioxidant and anticancer activities of Thai local vegetables. J Med Plants Res 3(5):443–449
Naqvi SA, Khan ZA, Hussain Z, Shahzad SA, Yar M, Ghaffar A, Mahmood N, Kousar S (2013) Antioxidant, antibacterial and antiproliferative activities of areal parts of Swertia chirata (Bush Ham) plant extracts using in vitro models. Asian J Chem 25(10):5448–5452
Nazir N, Zahoor M, Uddin F, Nisar M (2021) Chemical composition, in vitro antioxidant, anticholinesterase, and antidiabetic potential of essential oil of Elaeagnus umbellata Thunb. BMC Complement Med Ther 21:73. https://doi.org/10.1186/s12906-021-03228-y
Nimse SB, Pal DK (2015) Free radicals, natural antioxidants, and their reaction mechanisms. R Soc Chem Adv 5:27986–28006
Nunesa R, Pasko P, Tyszka-Czochara M, Szewczyk A, Szlosarczyk M, Carvalho IS (2016) Antibacterial, antioxidant and anti-proliferative properties and zinc content of five south Portugal herbs. Pharm Biol 55(1):114–123
Pushpa H, Ramya SN, Shetty SP, Ramesh DH (2015) Screening of antimicrobial, antioxidant and anticancer activity of Ruta graveolens . Adv Biol Res 9(4):257–264
Raj KD, Giri A (2021) Analysis of root extracts of Polycarpaea aurea in various organic solvents for their total polyphenolic content, total flavonoid content, and free radical scavenging activity. Plant Cell Biotechnol Mol Biol 22(31–32):138–147
Raj R, Chandrashekar KS, Maheshwari R, Polu PR, Pai V (2016) Pharmacognostical study of Syzygium caryophyllatum L. Res J Pharm Technol 9(10):1653–1657
Raja RR, Sreenivasulu M (2015) Medicinal plants secondary metabolites used in pharmaceutical importance-an overview. World J Pharm Pharmaceut Sci 4(4):436–447
Ranjit P, Veneetha Ch, Archana G (2016) Evaluation of total phenolics, flavonoids, and antioxidant activity of leaf extracts of Pimpenella tirupatensis . Int J Pharmaceut Sci Rev Res 41(1):58–63
Rice-Evans C, Miller NJ, Paganga G (1996) Structure –antioxidant activity relationship of flavonoids and phenolic acids. Free Radic Biol Med 20(7):933–956
Ritthiwigrom T, Laphookhieo S, Stephen G (2013) Chemical constituents and biological activities of Garcinia cowa Roxb. Maejo. Int J Sci Technol 7(02):212–231
Roengrit T, Wannanon P, Prasertsri P, Kanpetta Y, Sripanidkulchai B-O, Leelayuwat N (2014) Antioxidant and anti-nociceptive effects of Phyllanthus amarus on improving exercise recovery in sedentary men: a randomized crossover (double-blind) design. J Int Soc Sports Nutr 11:9
Saeed N, Khan MR, Shabbir M (2012) Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 12:221
Saha H, Srikkanth A, Sikchi S, Devi Rajeswari V (2015) Comparative evaluation of antimicrobial and anti-inflammatory activities of Ocimum sanctum, Phyllanthus niruri and Cadaba fruticosa : an in vitro Approach with emphasis on detection of their bioactive compounds using GC-MS. Int J Biol Chem 9(5):235–248
Salmerón-Manzano E, Manzano-Agugliaro F (2020) Bibliometric studies and worldwide research trends on global health. Int J Environ Res Public Health 17(16):5748. https://doi.org/10.3390/ijerph17165748 .
Scartezzeni P, Speroni E (2000) Review of some plants of Indian traditional medicine with anti-oxidant activity. J Ethnopharmacol 71(1–2):23–43
Schrader M, Fahimi HD (2006) Peroxisomes and oxidative stress. Biochem Biophys Acta 1763(12):1755–1766
Sen A, Batra A (2013) The study of in vitro and in vivo antioxidant activity and total phenolic content of Phyllanthus amarus schum . & Thonn. A medicinally important plant. Int J Pharm Pharmaceut Sci 5(3):942–947
Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, Prakash MA, Nigam M, El Rayess Y, Beyrouthy ME, Polito L, Iriti M, Martins M, Martorell M, Docea AO, Setzer WN, Calina D, Cho WC, Sharifi-Rad J (2020) Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol 11:694. https://doi.org/10.3389/fphys.2020
Sravanthi PSS, Giri A (2021a) In vitro cytotoxic activity of Phyllanthus amarus Schum. & Thonn. World J Biol Pharm Health Sci 06(02):034–042
Sravanthi PSS, Giri A (2021b) Phytochemicals and their antimicrobial activity: an update on their mode of action. Int J Clin Exp Med Res 5(1):41–69
Sravanthi PSS, Padmavathi TVS, Giri A (2016a) Metabolic fingerprinting of root, stem and leaf extracts of Phyllanthus amarus . J Phytol 8:17–21
Sravanthi PSS, Padmavathi TVS, Giri A (2016b) Pharmacological potential of an important medicinal plant Phyllanthus amarus Schum. & Thonn. Int J Adv Res Sci Technol (IJARST) 5(2):577–582
Sravanthi PSS, Padmavathi TVS, Giri A (2016c) Evaluation of anti-oxidant potential of root, stem and leaf extracts of Phyllanthus amarus . Int J Adv Res Sci Technol (IJARST) 5(3):653–661
Sravanthi PSS, Padmavathi TVS, Giri A (2016d) Analysis of bioactive compounds and antimicrobial screening of Phyllanthus amarus Schum. and Thonn. Int J Adv Res Sci Technol (IJARST) 5(2):595–603
Sravanthi SSP, Giri A, Lakshmi MV, Chakravarth B (2017) In silico molecular docking approach for anticancer analysis of bioactive compounds from P hyllanthus amarus dimethyl formamide leaf extracts. Int J Res Cult Soc 2017:152–163
Tan BL, Norhaizan ME, Liew WPP, Sulaiman Rahman H (2018) Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol 9:1162. https://doi.org/10.3389/fphar.2018.01162
Veeru P, Kishor MP, Meenakshi M (2009) Screening of medicinal plant extracts for antioxidant activity. J Med Plants Res 3(8):608–612
Vikneswaran M (2008) Phytochemical, pharmacological and pharmacokinetic studies of Phyllanthus niruri Linn. Lignans as potential anti-hyperuricemic agents. University Sains Malaysia, Penang
Vivek KR, Kumar S, Shashidhara S, Anitha S, Manjula A (2011) Comparison of the antioxidant capacity of an important hepatoprotective plants. Int J Pharmaceut Sci Drug Res 3(1):48–51
Welz AN, Emberger-Klein A, Menrad K (2018) Why people use herbal medicine: insights from a focus-group study in Germany. BMC Complement Altern Med 18:92. https://doi.org/10.1186/s12906-018-2160-6
Wink M (2015) Modes of action of herbal medicines and plant secondary metabolites. Medicines 2(3):251–286
Yogeswari S, Ramalakshmi S, Neelavathy R, Muthumary J (2012) Identificationand comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Glob J Pharmacol 6(2):65–71
Zhang QW, Lin LG, Ye WC (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 13:e20. https://doi.org/10.1186/s13020-018-0177-x
Download references
This research received no external funding.
Author information
Authors and affiliations.
Department of Botany, SVRK (M) GDC, Nidadavole, W.G.DT., Nidadavole, Andhra Pradesh, India
S. S. Sravanthi Pammi
Centre for Biotechnology, Institute of Science and Technology, Jawaharlal Nehru Technological University Hyderabad, Kukatpally, Hyderabad, 500 085, India
Bastipati Suresh & Archana Giri
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to S. S. Sravanthi Pammi .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Pammi, S.S.S., Suresh, B. & Giri, A. Antioxidant potential of medicinal plants. J. Crop Sci. Biotechnol. 26 , 13–26 (2023). https://doi.org/10.1007/s12892-022-00159-z
Download citation
Accepted : 21 May 2022
Published : 21 June 2022
Issue Date : January 2023
DOI : https://doi.org/10.1007/s12892-022-00159-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medicinal plants
- Secondary metabolites
- Phytochemicals
- Antioxidant activity
- Mode of action
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications
Deepak m kasote, surendra s katyare, mahabaleshwar v hegde, hanhong bae.
- Author information
- Article notes
- Copyright and License information
✉ Corresponding author: Hanhong Bae, [email protected]
Competing Interests: The authors have no competing interests to declare.
Received 2015 Mar 10; Accepted 2015 Apr 17; Collection date 2015.
Oxidative stress has been identified as the root cause of the development and progression of several diseases. Supplementation of exogenous antioxidants or boosting endogenous antioxidant defenses of the body is a promising way of combating the undesirable effects of reactive oxygen species (ROS) induced oxidative damage. Plants have an innate ability to biosynthesize a wide range of non-enzymatic antioxidants capable of attenuating ROS- induced oxidative damage. Several in vitro methods have been used to screen plants for their antioxidant potential, and in most of these assays they revealed potent antioxidant activity. However, prior to confirming their in vivo therapeutic efficacy, plant antioxidants have to pass through several physiopharmacological processes. Consequently, the findings of in vitro and in vivo antioxidant potential assessment studies are not always the same. Nevertheless, the results of in vitro assays have been irrelevantly extrapolated to the therapeutic application of plant antioxidants without undertaking sufficient in vivo studies. Therefore, we have briefly reviewed the physiology and redox biology of both plants and humans to improve our understanding of plant antioxidants as therapeutic entities. The applications and limitations of antioxidant activity measurement assays were also highlighted to identify the precise path to be followed for future research in the area of plant antioxidants.
Keywords: Antioxidant activity, pharmacology, plants, prooxidants, secondary metabolites
Introduction
Antioxidants significantly delay or prevent oxidation of oxidizable substrates when present at lower concentrations than the substrate 1 . Antioxidants can be synthesized in vivo (e.g., reduced glutathione (GSH), superoxide dismutase (SOD), etc.) or taken as dietary antioxidants 1 , 2 . Plants have long been a source of exogenous (i.e., dietary) antioxidants. It is believed that two-thirds of the world's plant species have medicinal importance, and almost all of these have excellent antioxidant potential 3 . The interest in the exogenous plant antioxidants was first evoked by the discovery and subsequent isolation of ascorbic acid from plants 4 . Since then, the antioxidant potential of plants has received a great deal of attention because increased oxidative stress has been identified as a major causative factor in the development and progression of several life threatening diseases, including neurodegenerative and cardiovascular disease. In addition, supplementation with exogenous antioxidants or boosting of endogenous antioxidant defenses of the body has been found to be a promising method of countering the undesirable effects of oxidative stress 5 .
There are currently approximately 19 in vitro and 10 in vivo methods of assessing antioxidant activity that are commonly applied for evaluation of the antioxidant activity of plant samples 6 . In most of these in vitro assays plant samples showed potent antioxidant activity. This is likely due to their innate ability to synthesize non-enzymatic antioxidants such as ascorbic acid and glutathione, as well as secondary metabolites such as phenolic compounds.
Despite many plants being reported to have antioxidant potential by in vitro assays, only a few of these antioxidant activities have been confirmed or investigated in vivo 7 . In vitro assays are generally used to confirm the antioxidant activity of plant samples within particular reaction systems; accordingly, the relevance of the findings of these assays to in vivo systems is uncertain 8 . Moreover, several phytochemicals have been found to possess antioxidant activity within in vitro assays. However, only a few of these have been shown to be therapeutically useful under in vivo conditions due to their interference with physiopharmacological processes such as absorption, distribution, metabolism, storage and excretion. Nevertheless, phytochemicals are being screened for their in vitro antioxidant activity, and the results of these studies are then directly extrapolated to their therapeutic usefulness. This malpractice may raise fundamental questions about the significance of plants as exogenous sources of antioxidants and their therapeutic efficacies. Accordingly, in the present article, we briefly reviewed the physiology and redox biology of both plants and humans. In addition, the applications and limitations of antioxidant activity measurement assays are discussed 6 , 7 . The information provided herein will enable correct interpretation of the findings of plant antioxidant potential assessment studies based on both in vitro and in vivo assays.
Why do all plants have antioxidant potential?
Chloroplasts and mitochondria are the two main powerhouses and sites of reactive oxygen species (ROS) generation within plant cells. These materials are also involved in maintenance of a fine balance between energy linked functions and control of ROS production. Peroxisomes, single membrane-bound subcellular organelles, are a third important site of production of ROS such as hydrogen peroxide (H 2 O 2 ), superoxide (O 2 ● - ) and nitric oxide (NO ● ) within plant cells. Peroxisomes contain basic enzymatic constituents such as catalase (CAT), as well as hydrogen peroxide (H 2 O 2 )-producing flavin oxidases 9 . Within the plant cell, ROS generation occurs at photosystem I and II (PS I and PS II) of the chloroplasts, membrane and matrix of the peroxisome, and complex I, ubiquinone and complex III of the mitochondrial electron transport chain (ETC) 10 . Under normal physiological conditions, there is electron slippage from PS I and PS II of the chloroplasts, membrane of mitochondrial ETC and peroxisome. These electrons later react with molecular oxygen to produce superoxide radical (O 2 -● ). The superoxide radical is subsequently converted to hydroperoxyl radical (HO 2 ● ) and finally to H 2 O 2 11 - 13 . Similar to ROS, reactive nitrogen species (RNS) such as the nitric oxide radical (NO • ) and peroxinitrite (ONOO-) are also formed in various compartments of the cell including the chloroplasts, mitochondria and peroxisomes 14 . The third type of free radical, reactive sulfur species (RSS), are reportedly formed from thiols by reaction with ROS 15 . The overall process of free radicals generation is summarized in Fig. 1 . These free radicals are constantly produced in the subcellular organelles of living cells . Most of the time, the production of free radicals is genetically planned, since they function as signaling molecules 12 , 16 . However, overproduction of free radicals can also sometimes damage biomolecules such as DNA, proteins and lipids.
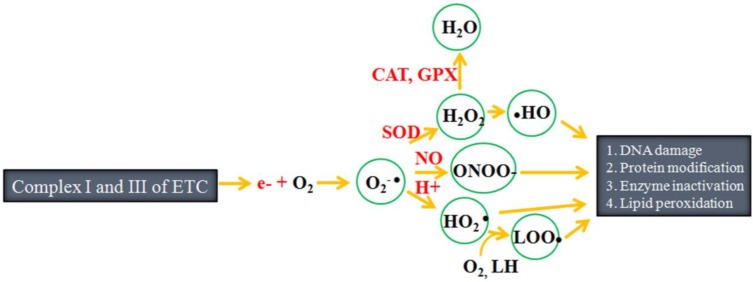
Outline of free radical production: During electron transfer, approximately 1-2% of the electrons slip from complex I and III of the electron transfer chain (ETC), after which they react with molecular oxygen to form free radicals such as superoxide anion (O 2 -● ), hydroperoxyl radical (HO 2 ● ), hydroxyl radical, hydrogen peroxide (H 2 O 2 ), hydroperoxyl radical ( ● OH), peroxynitrite (ONOO - ) and lipid peroxyl radical (LOO ● ). These free radicals target biomolecules such as DNA, protein and lipids, ultimately damaging them. SOD refers to superoxide dismutase, CAT refers to catalase and GPX refers to glutathione peroxidase. (Adapted from Carocho and Ferreira, 13 and Lü et al. 15 ).
Plants have efficient complex enzymatic and non-enzymatic antioxidant defense systems to avoid the toxic effects of free radicals. Enzymatic systems include SOD, catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) 7 , while non-enzymatic systems consist of low molecular weight antioxidants (ascorbic acid, glutathione, proline, carotenoids, phenolic acids, flavonoids, etc.) and high molecular weight secondary metabolites such as tannins (Fig. 2 ).
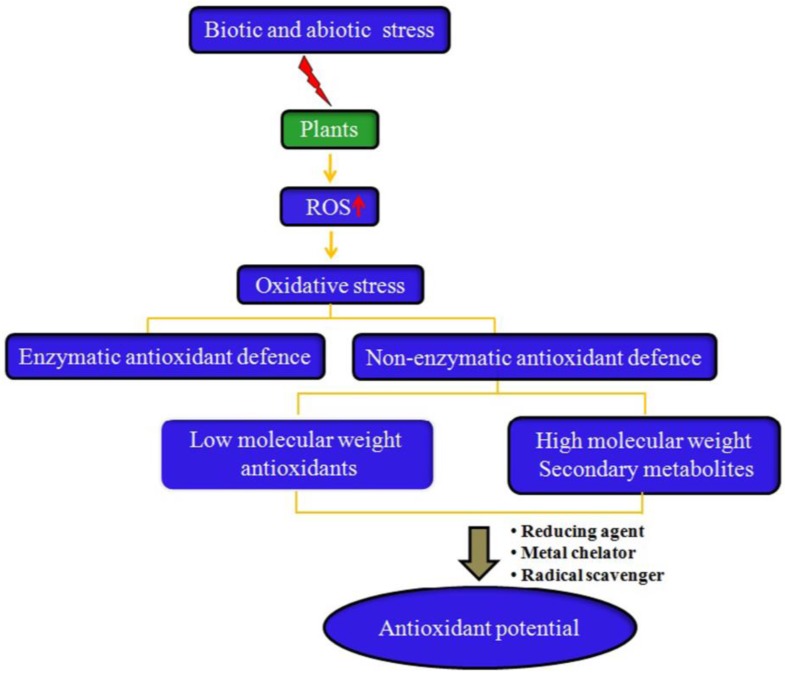
Why do all plants have antioxidant activity? Plants have an innate ability to synthesize non-enzymatic antioxidants. However, under biotic and abiotic stress conditions, the production of reactive oxygen species (ROS) increases in the plants, resulting in induction of oxidative stress. In response to increased oxidative stress, plants augment the production and accumulation of several low molecular weight antioxidants (e.g., vitamin C, vitamin E, phenolic acids, etc.) and high molecular antioxidant secondary metabolites such as tannins, which confer antioxidants to most plants under in vitro studies by functioning as free radical scavengers, reducing agents, and metal chelators.
There may be two main reasons for the synthesis and accumulation of these non-enzymatic antioxidants by plants. First, the genetic make-up of plants imparts them with an innate ability to synthesize a wide variety of phytochemicals to perform their normal physiological functions and/or protect themselves from microbial pathogens and animal herbivores. Another reason for the synthesis of reductant phytochemicals could be the natural tendency of plants to respond to environmental stress conditions.
Plants synthesize low molecular weight antioxidants such as glutathione and ascorbate within the chloroplast stroma and cytosol using NADPH as the ultimate electron donor 11 . These low molecular weight antioxidants function as redox buffers that interact with numerous cellular components and influence plant growth and development by modulating processes from mitosis and cell elongation to senescence and death 17 . In addition, these antioxidants may influence gene expression associated with biotic and abiotic stress responses to maximize defense. Vitamin C (ascorbic acid/ascorbate) is generated during aerobic metabolism, after which it reacts rapidly with O 2 -● , singlet oxygen and ozone (chemically), and H 2 O 2 (enzymatically) through ascorbate peroxidase to neutralize their toxic effects. Vitamin C also helps regenerate antioxidant pigments, carotenoids (carotenes and xanthophylls), and vitamin E. Glutathione is a redoxactive molecule that can be present in a reduced form (GSH) or an oxidized disulfide form (GSSG) and plays important roles in biosynthetic pathways, detoxification, antioxidant biochemistry and redox homeostasis 18 , 19 . GSSG is reduced to GSH by the enzyme glutathione reductase, which requires NADPH as the reducing power. GSH acts as an anti-oxidant by quenching reactive oxygen species and is involved in the ascorbate-glutathione cycle, which eliminates damaging peroxides 20 . Plants also produce tocopherols (vitamin E) that act as important liposoluble redox buffer systems . Vitamin E, which is generally synthesized in chloroplasts and protoplastids, is located in the membranes of cells. This compound is a major singlet oxygen scavenger that provides protection against lipid peroxidation 17 , 21 .
Plants also synthesize and accumulate a range of low and high molecular weight secondary metabolites that play important roles in ROS metabolism and avoidance of uncontrolled oxidation of essential biomolecules. These metabolites are also important to adaptation of plants to environmental fluctuations 22 . Secondary metabolites provide passive and active resistance. In passive resistance, metabolites are continuously available, despite the presence of stressors, whereas in active resistance , metabolites are produced in response to specific stressors 23 . These metabolites are synthesized through basic pathways, such as the glycolysis or shikimic acid pathways, which further branch out based on cell type, developmental stage and environmental cues. Secondary metabolites are generally derived from primary metabolites such as amino acids and carbohydrates via methylation, hydroxylation and glycosylation 24 .
Higher plants survive in constantly fluctuating environments, due to their highly regulated and flexible metabolism 25 . Under normal physiological conditions, the increase in free radical production is relatively small and housekeeping antioxidant capacity is sufficient to maintain redox homeostasis 26 . The metabolic pathways of plants are sensitive to abiotic and biotic stress conditions such as high light intensity, heat, drought, anoxic conditions and pathogen attack, and it has been reported that there is an approximately 3 to 10 fold increase in free radicals production under stress conditions 14 , 25 , 27 .
The ratio of GSH to GSSG has been shown to decrease due to the oxidation of reduced glutathione during detoxification of reactive oxygen species (ROS) in response to abiotic stresses 28 . Moreover, plants increase the activity of GSH biosynthetic enzymes and glutathione levels in response to both abiotic and biotic stresses 29 . Similar to glutathione, biosynthesis and recycling of ascorbic acid has been found to increase in response to various abiotic stresses within mutant and transgenic plant species 30 , 31 . Vitamin E deficiency has also been shown to retard growth and change responses to abiotic stress conditions. In addition, increased vitamin E content has been shown to diminish detrimental effects of environmental stress in plants 32 .
Some secondary antioxidant metabolites occur constitutively, while others are formed in response to biotic and abiotic stress conditions 33 , 34 . The accumulation of phenolic compounds along with enhancement of phenylopropanoid metabolism has been observed under different environmental stress conditions 35 . In plants, phenolics can act as antioxidants by donating electrons to guaiacol-type peroxidases for the detoxification of H 2 O 2 produced under stress conditions 36 . Phenolics also provide protection against UV radiation through their potent radical scavenging ability. In addition, they function as enzyme inhibitors and feeding deterrents for herbivores while providing resistance against pathogens 37 . Synthesis of flavonoids is known to be induced by UV stress, heavy metals toxicity, or low temperature and low nutrient conditions, which might attributed to their UV-absorbing, radical scavenging and metal cheating ability 35 , 38 , 39 . UV-B radiation was found to affect the production of various high molecular secondary metabolites such as tannins and lignin 40 . Moreover, plants growing in tropical and high-altitude conditions have been shown to contain a higher proportion of flavonoids than those growing in temperate conditions owing to overexposure to light or UV radiation 41 . Biotic stress like wounding has been found to induce phenolic metabolism such as increased synthesis of phenolic compounds 42 . Tannins are reportedly useful for plant leaf defense against insect herbivores 43 . Similar to phenolics, an increase in total indole alkaloid content in the shoots and roots of Catharanthus roseus has been observed under drought-induced stress 44 . Alkaloids generally provide protection to plants against microbial or herbivore attack and UV-radiation 45 , 46 . It has also been reported that monoterpenes and isoprenes are emitted at higher rates under high temperature 47 .
Secondary metabolites as antioxidants
Plant metabolism is mainly classified as primary or secondary. Compounds produced through primary metabolism, which are generally referred to as primary metabolites; include sugars, fatty acids, amino acids and nucleic acids. Primary metabolites are required for maintenance of plant cells 48 , while secondary metabolites are essential to the normal growth, development and defense of plants.
To date, thousands of different types of secondary metabolites have been identified in plants 23 . Chemically, these compounds are either nitrogen-containing (alkaloids) or nitrogen-deficient (terpenoids and phenolics) 46 . Nearly 20% of plant species accumulate alkaloids, which mainly include terpenoid indole alkaloids, tropane alkaloids, and purine alkaloids 49 . However, under in vitro antioxidant measurement assay conditions, the radical scavenging potential of alkaloids is reportedly moderate to nonexistent. Terpenoids comprise another large family of secondary metabolites, consisting of over 40,000 different compounds 50 . Monoterpenes, sesquiterpenes and diterpenes have been found to possess notable antioxidant activity in different in vitro assays. However, most of these activities have no physiological relevance 51 . Tetraterpenes and carotenoids have been shown to possess potent antioxidant activity within both in vivo and in vitro studies 52 ; however, some valuable carotenoids such as beta-carotene showed prooxidant effects at high concentration and oxygen pressure 53 . Among all secondary metabolites, phenolic antioxidants appear to be the most important since they have shown promising antioxidant activity in both in vivo and in vitro investigations. Plant phenolics are mainly classified into five major groups, phenolic acids, flavonoids, lignans, stilbenes and tannins 54 - 56 . Phenolic compounds generally possess one or more aromatic rings with one or more hydroxyl groups. It has commonly been assumed that the antioxidant capacity of phenolics will increase with the number of free hydroxyls and conjugation of side chains to the aromatic rings 57 . Flavonoids and phenolic acids, the largest classes of plant phenolics, are biosynthetically derived from the acetate and shikimate pathways, as well as the shikimate pathway from phenylalanine or tyrosine 58 . Phytochemicals from these classes were found to have excellent antioxidant activity in both in vitro and in vivo investigations. Moreover, they are known to interact with other physiological antioxidants such as ascorbate or tocopherol and to synergistically amplify their biological effects 59 . Flavonoids and phenylopropanoids are also oxidized by peroxidase, and act as H 2 O 2 scavengers 35 , 60 . Under experimental conditions, the antioxidant potential of plant phenolics is always linked to their electron donation, reducing power and metal ion chelating ability 61 .
Methods used for assessment of antioxidant potential of plants
In vitro assays.
In ethanopharmacological and nutraceutical investigations, in vitro antioxidant activity assessment methods are often used to screen and confer antioxidant potential to plants or their phytochemicals and sometimes to understand the probable mechanism of action of plant antioxidants 62 . In the case of medicinal plants, these assays are used to confer free radical scavenging activity to plants, which in turn has great importance in understanding the role of plants in minimizing the oxidative stress linked pathophysiology of diseases. There are several in vitro assays used to measure and confer antioxidant activity to plants (Table 1 ); however, each of these has its own limitations regarding applicability . Therefore , multiple assay strategies have frequently been adapted to confer antioxidant potential. In these assays, plants are generally assessed for their function as reducing agents, hydrogen donors, singlet oxygen quenchers or metal chelators, after which they are classified as primary (chain-breaking) and secondary (preventive) antioxidants. Primary antioxidants act by donating a hydrogen atom, while secondary antioxidants function via binding of metal ions capable of catalyzing oxidative processes and scavenging oxygen, absorbing UV radiation, inhibiting enzymes or decomposing hydroperoxides 66 .
In vitro assays commonly used to screen antioxidant activity of plants.
Based on the inactivation mechanism involved, antioxidant activity assessment methods are classified into hydrogen atom transfer (HAT) and electron transfer (ET) reaction-based methods. Bond dissociation energy and ionization potential are two major factors that determine the mechanism and efficiency of antioxidants 63 . HAT-based methods measure the ability of an antioxidant to scavenge free radicals via hydrogen donation to form stable compounds. While these methods are more relevant to the radical chain-breaking antioxidant capacity, SET-based methods measure the ability of an antioxidant to transfer one electron to reduce any compound, including metals, carbonyls, and free radicals 63 , 67 . Total radical trapping antioxidant parameter (TRAP), oxygen radical absorbance capacity (ORAC), lipid peroxidation inhibition capacity (LPIC) and carotene or crocin-bleaching assays are HAT-based methods . Other commonly used antioxidant activity assessing methods such as ferric reducing antioxidant power (FRAP) and copper reduction assay involve SET mechanisms 64 . However, some methods, such as 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and {2,2'-azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)} (ABTS), involve both HAT and SET mechanisms 67 .
In vivo assays
Within in vivo assays, plant antioxidants are generally assessed for their effects on the activity of endogenous antioxidant enzymes or oxidative damage biomarkers before and after induction of oxidative stress in experimental animals. Some of these commonly used methods directly evaluate the enzymatic activity of endogenous antioxidants such as SOD, CAT, GPx and GR, while other methods involve quantification of oxidative damage biomarkers. The formation of specific end products resulting from interaction of ROS with biologically important macromolecules such as DNA, protein and lipids is measured by quantifying oxidative damage biomarker methods. DNA damage is determined by measuring the 8-hydroxydeoxyguanosine content. Carbonyl and aldehyde (e.g. malondialdehyde) contents are measured as markers of protein and lipid oxidation, respectively 6 , 68 .
Therapeutic relevance and in vivo behavior of plant antioxidants
Similar to plants, the human body is constantly exposed to oxidants and/or free radicals generated during physiological processes such as mitochondrial respiration. In plants, the production of free radicals increases during biotic and abiotic stresses, whereas the load of free radicals in humans increases under pathophysiological conditions such as inflammation, metabolism of foreign compounds, and radiation 69 . In animal cells, free radicals are generated at the ETC of mitochondria, Ero1 and cytochrome P-450 enzymes of the endoplasmic reticulum (ER), and at the plasma membrane (at the NADPH oxidases) and inside (at the flavin oxidases) of peroxisomes 70 . Mitochondria produce more than 90% of the cellular energy through oxidative phosphorylation, which involves the tricarboxylic acid cycle and ETC. However, about 1-2% of the electrons slip through complexes I and III of the ETC, after which they react with molecular oxygen to sequentially form ROS such as O 2 -● , HO 2 ● and H 2 O 2 5 , 11 - 13 . The peroxisome is another major site of oxygen consumption within animal cells. In this site, electrons removed from various metabolites are used to reduce O 2 to H 2 O 2 , which is then converted to H 2 O. The peroxisome plays a key role in both the production and scavenging of ROS in the cell, mainly in the form of H 2 O 2 . The respiratory pathway in peroxisomes is not coupled to oxidative phosphorylation; therefore, it does not produce ATP. However, free energy is released in the form of heat 71 . It is well known that peroxisomes in plant cells contain a functional ascorbate-glutathione cycle; however, relatively little is known about the presence of this non-enzymatic antioxidant inside mammalian peroxisomes 72 . While ER is responsible for much of a cell's protein synthesis and folding, it also produces ROS as a byproduct 73 .
As in the case of the plants, free radicals also act as signaling molecules within animal cells. Specifically, they play an important role in apoptosis, gene expression and ion transportation 15 . The human body also has an efficient antioxidant defense system that maintains a balance between free radical production and oxidative stress through enzymatic and non-enzymatic antioxidant defenses. Enzymatic antioxidant defenses include SOD, CAT, glutathione GPx, GR and glucose-6-phosphate dehydrogenase, while non-enzymatic antioxidant defenses primarily consist of vitamin A, coenzyme Q (Q10), uric acid and glutathione 13 .
Peroxisomes and mitochondria are interconnected and in close contact with the ER to maintain various metabolic and signaling pathways 74 . However, dysfunction in any of these organelles leads to overproduction of free radicals, which exerts a toxic effect on biomolecules such as DNA, proteins and lipids, which leads to deregulation of redox-sensitive metabolic and signaling pathways and pathological conditions 71 .
It should be noted that environmental and complex genetic causes result in almost all cells overproducing life threatening free radicals in their early or late stages of life. The human body has efficient enzymatic antioxidant defense; however, its non-enzymatic antioxidant defense is less evolved than that of plants. This may be due to its low oxygen exposure physiology. Hence, it has been assumed that humans must constantly take in dietary antioxidants to keep the levels of free radicals in the body low.
Plants have always been a common source of food and medicines, either in the form of traditional preparations or as pure active principles 75 . Most of the observed therapeutic effects of plants have been linked to their potent antioxidant activity. We previously proposed that antioxidant activity based healing of diseases or maintenance of a healthy lifestyle could be the scientific basis of traditional herbal medicines such as those used in Ayurveda 76 . It has been suggested that free radicals are involved in the pathology of more than 50 human diseases, including aging 77 . However, it is also important to consider that free radicals are not harmful at all times; rather, their toxicity depends on several factors including type of ROS/RNS, their concentration and localization, and the kinetics of production and elimination 70 .
Despite the controversies regarding whether plant antioxidants are good or bad, supplementation of external antioxidants or boosting endogenous antioxidant defenses of the body is known to be a promising method to improve the free radical linked pathophysiology of diseases. Plant antioxidants such as ascorbic acid and flavonoids have been shown to be the best exogenous antioxidants. Indeed, these compounds not only restrain ROS production by scavenging free radicals, but also help boost endogenous antioxidant defenses of the body (Fig. 3 ). Nevertheless, the usefulness of endogenous antioxidants as therapeutic agents still remains an issue that must be carefully considered 78 .
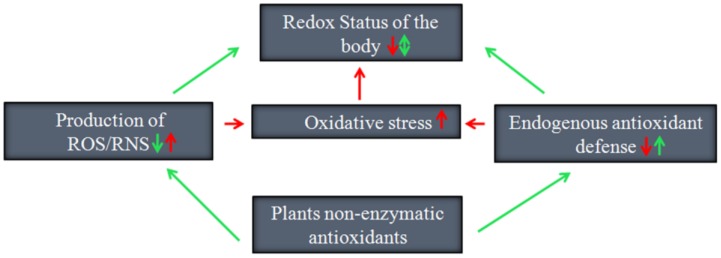
Promising ways by which plant non-enzymatic antioxidants preserve the redox status of the human body.
While both plants and humans have somewhat similar redox biology, the physiology of humans is distinct from that of plants. It is important to consider the fact that plants produce antioxidants for their own requirements to perform specific functions. In the human body, plant antioxidants have to pass through several physiological processes including absorption, distribution, metabolism, storage and excretion before being able to achieve the expected therapeutic effect. As a result, most good plant antioxidants are unable to achieve the desired outcome because they generally have low bioavailability and some can exert pro-oxidant effects under in vivo conditions 79 , 80 .
Similar to lower vertebrates, humans cannot synthesize Vitamin C due to the lack of gulonolactone oxidase 81 . Accordingly, vitamin C is regarded as one of the most important dietary antioxidants that helps to decrease free-radical-mediated damage. Vitamin C is readily absorbed by active transport in the intestine and functions as an antioxidant by donating two of its electrons, which prevents other compounds from being oxidized 81 , 82 . Vitamin C has been shown to be an excellent antioxidant under both in vitro and in vivo study conditions. However, in the presence of catalytic metal ions, vitamin C can also function as a pro-oxidant 83 . Specifically, vitamin C has been found to be capable of converting Fe 3+ into Fe 2+ , which subsequently reacts with oxygen or hydrogen peroxide to form superoxide and hydroxyl radicals that can subsequently damage biomolecules 84 . However, the pro-oxidant properties of vitamin C are believed to be dependent on dose as well as the availability of catalytic metal ions 85 . In addition, the antioxidant or pro-oxidant behavior of vitamin C has been shown to depend on the vitamin E status of the body 86 . Vitamin E is another useful and powerful plant antioxidant that is generally present in lipid structures of cells. This compound reacts with peroxyl radicals to inhibit the propagation of lipid peroxidation 87 . Similar to vitamin C, vitamin E also shows pro-oxidant effects at high concentration. Vitamin E has also been shown to react with free radicals to become a reactive radical, while it functions as a prooxidant in the absence of co-antioxidants 13 .
Under in vivo conditions, the antioxidant potential of polyphenols is predominately dependent on their concentrations in the bloodstream after absorption from the gastrointestinal tract, as well as their modifications during metabolism. The chemical structures of polyphenols will also influence the in vivo antioxidant potential of polyphenols, as they determine the conjugation reactions with methyl, sulfate or glucuronide groups and the nature and amounts of metabolites formed by the gut microflora absorbed at the colon level 88 .
Flavonoids are the most abundant dietary polyphenols, with over 5000 reported to date 89 , 90 . Flavonoids are classified into six major subclasses, flavones, flavonols, flavanones, catechins or flavanols, anthocyanidins and isoflavones. In plants, most flavonoids are attached to sugars (glycosides), although they are occasionally found as aglycones 89 . Expect for some flavan-3-ols and proanthocyanidins, most flavonoids are not completely absorbed and reach the circulatory system owing to their glycoside conjugates 91 . Occasionally, major fractions of absorbed flavonoids are metabolized to conjugates or further metabolized in the colon, where they produce a wide array of low molecular weight aromatic acids such as phenylvaleric, phenylpropionic, phenylacetic and benzoic acids as a result of their antioxidant potency being reduced 92 - 94 . Moreover, flavonoids and their in vivo metabolites have been reported to exert other potential biological activities than conventional hydrogen-donating antioxidants, such as the ability to act as signaling molecules in cells through actions in the protein kinase and lipid kinase signaling pathways 94 , 95 . In addition to their notable antioxidant behavior, polyphenols have pro-oxidant properties. Indeed, it has been reported that plant polyphenols may increase oxidative damage in vivo via interactions with transition metal ions that increase their ability to form free radicals from peroxides 96 .
Carotenoids, which are abundant in fruits and vegetables, scavenge peroxyl radicals and act predominantly as antioxidants 97 . The antioxidant potential of dietary carotenoids such as beta-carotene and lycopene in biological systems is dependent on a number of factors, including the presence of other co-antioxidants. It has also been reported that carotenoids may lose their effectiveness as antioxidants at high concentrations or at high partial pressures of oxygen 98 .
Strategy for plant antioxidant potential measurement
In vitro antioxidant potential assessment methods do not provide exact therapeutic implications of plant antioxidants. Moreover, the antioxidant potential of plants or their phytochemicals is influenced by several factors under in vivo conditions, including gut absorption, metabolism, bioavailability, and presence of co-antioxidants and transition metal ions. Consequently the results of in vivo antioxidant assessment studies of plant antioxidants are not consistent 62 . Hence, there is a need to develop an expansive study strategy that will include a set of in vitro and in vivo experiments to provide more accurate therapeutic values to plant antioxidants.
One commonly suggested strategy is that both in vitro and in vivo antioxidant assessment studies be conducted simultaneously to confer therapeutic antioxidant potential to plants or their components. Holst and Williamson 99 proposed that in vitro plant antioxidant assessment studies be driven by in vivo results, and not vice versa. They further suggested that once a phytochemical is shown to exert an effect in vivo , their mechanisms can be tested in vitro to avoid disappointments when testing in vitro concepts in vivo . It is believed that the proposed antioxidant activity assessment studies would be more suitable for investigation of antioxidant activity of flavonoids and lignans, as these phytochemicals are generally metabolized to low molecular antioxidants in the body. Most ingested flavonoids have been shown to be extensively degraded to various phenolic acids, which could have radical scavenging ability 100 . Similarly, the lignan secoisolariciresinol diglucoside is metabolized to more powerful antioxidants such as secoisolariciresinol, enterodiol and enterolactone within the body 101 .
In line with the above plant antioxidant activity assessment study strategies, it is advisable disease pathophysiology targeted combined in vitro and in vivo antioxidant activity assessment study strategy to attribute more precise therapeutic value to individual or combined plant antioxidant entities. In this strategy, there is a need to first identify the major target of free radical linked disease pathophysiology such as mitochondrial dysfunction, which is an underlying cause of several degenerative diseases. Thereafter, plant extracts or antioxidants should be screened for low molecular antioxidants that could have potent radical scavenging activity in vitro and are able to cross blood brain barriers and reach the target by comparing them with proposed or known structural analogs of mitochondria-targeted antioxidants through in silico methods. Finally, these results should be validated by in vivo studies.
Conclusions
Over the past few decades, significant scientific information has been accumulated regarding plant redox biology and its antioxidant defense. However, this information has not been collectively discussed together with human redox biology and exogenous antioxidants metabolism, which is essential in understanding the therapeutic utility of plant antioxidants. Because of their high oxygen exposure physiology, plants may have more sites of ROS generation; therefore, they could evolve more efficient non-enzymatic antioxidant systems than humans. Plants synthesize and accumulate several non-enzymatic antioxidants such as ascorbic acid, glutathione and phenolics. Some of these antioxidants occur constitutively, while others are formed in response to abiotic and biotic stress conditions. Almost all plants or their phytochemicals exhibit some antioxidant activity under in vitro assays conditions. However, for in vivo studies, plant antioxidants have to pass through several physiopharmacological processes including absorption, distribution, metabolism, storage and excretion. Consequently, the antioxidant potential of plants or their phytochemicals is influenced by several factors in vivo , including gut absorption, metabolism, bioavailability, and the presence or absence of co-antioxidants and transition metal ions. Therefore, the results of the in vitro antioxidant potential assessment studies are often contradictory to those of in vivo studies. Nevertheless, without undertaking sufficient in vivo studies, the results of in vitro assays have been irrelevantly linked to the therapeutic applications of plant antioxidants. Hence, we proposed disease pathophysiology targeting combined in vitro and in vivo antioxidant activity to attribute more precise therapeutic value to individual or combined plant antioxidant entities.
Acknowledgments
This research was supported by a Yeungnam University Research Grant (214A345035).
Abbreviations
2,2'-Azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)
2,2-Diphenyl-1-picrylhydrazyl
Electron Transport Chain
Ferric Reducing Antioxidant Power
Glutathione Peroxidase
Glutathione Reductase
Reduced Glutathione
Oxidized Glutathione
Hydrogen Peroxide
Hydrogen Atom Transfer
Hydroperoxyl Radical
Lipid Peroxidation Inhibition Capacity
Nicotinamide Adenine Dinucleotide Phosphate
Nitric Oxide
Superoxide Anion
Peroxynitrile
Oxygen Radical Absorbance Capacity
Photosystem I
Photosystem II
Reactive Nitrogen Species
Reactive Oxygen Species
Reactive Sulfur Species
Superoxide Dismutase
Tricarboxylic Acid Cycle
Total Radical Trapping Antioxidant Parameter.
- 1. Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–50. doi: 10.1042/BST0351147. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Sies H. Oxidative stress: oxidants, antioxidants. Exp Physiol. 1997;82:291–95. doi: 10.1113/expphysiol.1997.sp004024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–33. [ Google Scholar ]
- 4. Szent-Giörgyi A. Lost in the twentieth century. Annu Rev Biochem. 1963;36:1–15. doi: 10.1146/annurev.bi.32.070163.000245. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Kasote DM, Hegde MV, Katyare SS. Mitochondrial dysfunction in psychiatric and neurological diseases: cause(s), consequence(s), and implications of antioxidant therapy. Biofactors. 2013;39:392–06. doi: 10.1002/biof.1093. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–52. doi: 10.1016/j.jsps.2012.05.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Chand S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res. 2009;3:981–96. [ Google Scholar ]
- 8. Badarinath AV, Rao KM, Chetty CMS, Ramkanth V, Rajan TVS, Gnanaprakash K. A review on in-vitro antioxidant methods: comparisons, correlations and considerations. Int J PharmTech Res. 2010;2:1276–85. [ Google Scholar ]
- 9. del Río LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB. Reactive oxygen species, reactive nitrogen species in peroxisomes. Production, scavenging, and, role in cell signaling. Plant Physiol. 2006;141:330–35. doi: 10.1104/pp.106.078204. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. doi: 10.1016/j.plaphy.2010.08.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol Plant. 1997;100:224–33. [ Google Scholar ]
- 12. Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–81. doi: 10.1146/annurev.arplant.58.032806.103946. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–60. doi: 10.1111/j.1582-4934.2009.00897.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. doi: 10.1016/s1360-1385(02)02312-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Foyer CH. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–75. doi: 10.1105/tpc.105.033589. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Meyer AJ, Hell R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res. 2005;86:435–457. doi: 10.1007/s11120-005-8425-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B. et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–84. doi: 10.1111/j.1365-3040.2011.02400.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Galant A, Preussm ML, Cameron JC, Jez JM. Plant glutathione biosynthesis: diversity in biochemical regulation and reaction products. Front. Plant Sci. 2011;2:45. doi: 10.3389/fpls.2011.00045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Jaleel CA, Riadh K, Gopi R, Manivannan P, Inès J, Al-Juburi HJ. et al. ZhaoAntioxidant defense response: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant. 2009;31:427–36. [ Google Scholar ]
- 22. Baier M, Dietz KJ. Chloroplasts as source and target of cellular redox regulation: a discussion on chloroplast redox signals in the context of plant physiology. J Exp Bot. 2005;56:1449–62. doi: 10.1093/jxb/eri161. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [ PubMed ] [ Google Scholar ]
- 24. Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell. 2004;16:2463–2480. doi: 10.1105/tpc.104.022897. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Shao HB, Chu LY, Shao MA, Jaleel CA, Mi HM. Higher plant antioxidants and redox signaling under environmental stresses. C R Biol. 2008;331:433–41. doi: 10.1016/j.crvi.2008.03.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Van Breusegem F, Dat J. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–90. doi: 10.1104/pp.106.078295. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35:259–70. doi: 10.1111/j.1365-3040.2011.02336.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Szalai G, Kellos T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul. 2009;28:66–80. [ Google Scholar ]
- 29. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Gao Q, Zhang L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol. 2008;165:138–148. doi: 10.1016/j.jplph.2007.04.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Wang Z, Xiao Y, Chen W, Tang K, Zhang L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol. 2010;52:400–09. doi: 10.1111/j.1744-7909.2010.00921.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Espinoza A, San Martín A, López-Climent M, Ruiz-Lara S, Gómez-Cadenas A, Casaretto JA. Engineered drought-induced biosynthesis of α-tocopherol alleviates stress-induced leaf damage in tobacco. J Plant Physiol. 2013;170:1285–94. doi: 10.1016/j.jplph.2013.04.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–89. [ Google Scholar ]
- 34. Bailey BA, Strem MD, Bae H, Antunez de Mayolo G, Guiltinan MJ. Gene expression in leaves of Theobroma cacao in response to mechanical wounding, ethylene, and/or methyl jasmonate. Plant Sci. 2005;168:1247–58. [ Google Scholar ]
- 35. Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J of Environ Stud. 2006;15:523–30. [ Google Scholar ]
- 36. Sakihama Y, Cohen MF, Grace SC, Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Bennett RN, Wallsgrove RM. Secondary metabolites in plant defence mechanisms. New Phytol. 1994;127:617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol. 2002;5:218–23. doi: 10.1016/s1369-5266(02)00256-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Rivero RM, Ruiz JM, García PC, López-Lefebre LR, Sánchez E, Romero L. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–21. doi: 10.1016/s0168-9452(00)00395-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Rozema J, van de Staaij J, Björn LO, Caldwell M. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol. 1997;12:22–28. doi: 10.1016/s0169-5347(96)10062-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Chappell J, Hahlbrock K. Transcription of plant defence genes in response to UV light and fungal elicitor. Nature. 1984;311:76–78. [ Google Scholar ]
- 42. Saltveit ME. Wound-induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol Tec. 2000;21:61–69. [ Google Scholar ]
- 43. Barbehenn RV, Peter Constabel C. Tannins in plant-herbivore interactions. Phytochemistry. 2011;72:1551–65. doi: 10.1016/j.phytochem.2011.01.040. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Jaleel CA, Manivannan P, Sankar B, Kishorekumar A, Gopi R, Somasundaram R. et al. Water deficit stress mitigation by calcium chloride in Catharanthus roseus: effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf B Biointerfaces. 2007;60:110–116. doi: 10.1016/j.colsurfb.2007.06.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. De Luca V, St Pierre B. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 2000;5:168–173. doi: 10.1016/s1360-1385(00)01575-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Patra B, Schluttenhofer C, Wu Y, Pattanaik S, Yuan L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochim Biophys Acta. 2013;1829:1236–47. doi: 10.1016/j.bbagrm.2013.09.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Loreto F, Förster A, Dürr M, Csiky O, Seufert G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 1998;21:101–07. [ Google Scholar ]
- 48. Kliebenstein DJ, Osbourn A. Making new molecules - evolution of pathways for novel metabolites in plants. Curr Opin Plant Biol. 2012;15:415–23. doi: 10.1016/j.pbi.2012.05.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Ziegler J, Facchini PJ. Alkaloid biosynthesis: metabolism and trafficking. Annu. Rev. Plant Biol. 2008;59:735–69. doi: 10.1146/annurev.arplant.59.032607.092730. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Aharoni A, Jongsma MA, Bouwmeester HJ. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005;10:594–02. doi: 10.1016/j.tplants.2005.10.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Baratta MT, Dorman HJD, Deans SG. Chemical composition, antimicrobial and antioxidative activity of laure, sage, rosemary, oregano and coriander essential oils. J Essent Oil Res. 1998;10:618–27. [ Google Scholar ]
- 52. Palozza P, Krinsky NI. Antioxidant effects of carotenoids in Vivo and in Vitro: An overview. Methods Enzymol. 1992;213:403–20. doi: 10.1016/0076-6879(92)13142-k. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Burton GW, Ingold KU. Beta-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–73. doi: 10.1126/science.6710156. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Myburgh KH. Polyphenol supplementation: benefits for exercise performance or oxidative stress? Sports Med. 2014;1:S57–70. doi: 10.1007/s40279-014-0151-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94. doi: 10.1093/aob/mcf118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Morgan JF, Klucas RV, Grayer RJ, Abian J, Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med. 1997;22:861–70. doi: 10.1016/s0891-5849(96)00426-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Dewick PM. The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids, in Medicinal Natural Products: A Biosynthetic Approach, 3rd Edition. Chichester, UK: John Wiley & Sons, Ltd; 2009. [ Google Scholar ]
- 59. Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998;854:435–42. doi: 10.1111/j.1749-6632.1998.tb09922.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Sakihama Y, Cohen MF, Grace SC, Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–59. [ Google Scholar ]
- 62. Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–98. doi: 10.1039/b009171p. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Karadag A, Ozcelik B, Saner S. Review of methods to determine antioxidant capacities. Food Anal. Methods. 2009;2:41–60. [ Google Scholar ]
- 64. Ou B, Woodill-Hampsch M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Huang D, Ou B, Prior R L. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. doi: 10.1021/jf030723c. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Kasote DM. Flaxseed phenolics as natural antioxidants. Int Food Res J. 2013;20:1797–04. [ Google Scholar ]
- 67. Prior R, L Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–02. doi: 10.1021/jf0502698. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol. 2007;21:111–27. doi: 10.1111/j.1472-8206.2006.00466.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Nordgren M, Fransen M. Peroxisomal metabolism and oxidative stress. Biochimie. 2014;98:56–62. doi: 10.1016/j.biochi.2013.07.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;63:1755–66. doi: 10.1016/j.bbamcr.2006.09.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta. 2012;1822:1363–73. doi: 10.1016/j.bbadis.2011.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Thoms S, Grønborg S, Gärtner J. Organelle interplay in peroxisomal disorders. Trends Mol Med. 2009;15:293–02. doi: 10.1016/j.molmed.2009.05.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63:965–81. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Hegde MV, Patil S, Bhalerao S. A philosophy for integration of ayurveda with modern medicine: A biochemist's perspective. Curr Sci. 2008;95:721–22. [ Google Scholar ]
- 77. Halliwell B. Drug antioxidant effects. Drugs. 1991;42:569–05. doi: 10.2165/00003495-199142040-00003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Halliwell B. Reactive species and antioxidants. redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–22. doi: 10.1104/pp.106.077073. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Halliwell B. Commentary: Vitamin C: Antioxidant or Pro-Oxidant In Vivo? Free Rad Res. 1996;25:439–54. doi: 10.3109/10715769609149066. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Bast A, Haenen GR. Ten misconceptions about antioxidants. Trends Pharmacol Sci. 2013;34:430–36. doi: 10.1016/j.tips.2013.05.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Putchala MC, Ramani P, Sherlin HJ, Premkumar P, Natesan A. Ascorbic acid and its pro-oxidant activity as a therapy for tumours of oral cavity -- a systematic review. Arch. Oral Biol. 2013;58:563–74. doi: 10.1016/j.archoralbio.2013.01.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Heo JH. et al. The possible role of antioxidant vitamin C in Alzheimer's disease treatment and prevention. Am J Alzheimers Dis Other Demen. 2013;28:120–25. doi: 10.1177/1533317512473193. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Kang MJ, Lee SS, Koh HC. Prooxidant properties of ascorbic acid in the nigrostriatal dopaminergic system of C57BL/6 mice. Toxicology. 2012;294:1–8. doi: 10.1016/j.tox.2012.01.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Rietjens IM, Boersma MG, Haanm Ld, Spenkelink B, Awad HM, Cnubben NH. et al. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol. 2002;11:321–33. doi: 10.1016/s1382-6689(02)00003-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826:443–57. doi: 10.1016/j.bbcan.2012.06.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Chen LH. Interaction of vitamin E and ascorbic acid (review) In Vivo. 1989;3:199–09. [ PubMed ] [ Google Scholar ]
- 87. Wagner BA, Buettner GR, Burns CP. Vitamin E slows the rate of free radical-mediated lipid peroxidation in cells. Arch Biochem Biophys. 1996;334:261–67. doi: 10.1006/abbi.1996.0454. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–52. doi: 10.3390/molecules15107313. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Donovan JL, Manach C, Faulks RM, Kroon PA. Eds. absorption and metabolism of dietary plant secondary metabolites, in plant secondary metabolites: occurrence, structure and role in the human diet, Blackwell Publishing Ltd, Oxford, UK; 2006. [ Google Scholar ]
- 92. Scalbert A, Morand C, Manach C, Rémésy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002;56:76–82. doi: 10.1016/s0753-3322(02)00205-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic Res. 2005;39:457–69. doi: 10.1080/10715760500053610. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr. 2005;81:268S–76S. doi: 10.1093/ajcn/81.1.268S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–49. doi: 10.1016/j.freeradbiomed.2004.01.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Decker EA. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997;55:396–98. doi: 10.1111/j.1753-4887.1997.tb01580.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem. 2013;70:102–10. doi: 10.1016/j.ejmech.2013.09.054. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73–82. doi: 10.1016/j.copbio.2008.03.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Prasad K. Antioxidant Activity of Secoisolariciresinol Diglucoside-derived Metabolites, Secoisolariciresinol, Enterodiol, and Enterolactone. Int J Angiol. 2000;9:220–25. doi: 10.1007/BF01623898. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (519.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
COMMENTS
As shown in Table 3, the antioxidant activities of many food and medicinal plants have been widely estimated, e.g., fruits, vegetables, cereal grains, edible and wild flowers, macro-fungi, medicinal plants, spices, etc., and the varieties showing strong antioxidant activities were displayed in Table 3 based on a combinative consideration of the ...
Mar 1, 2013 · Over the years, research on antioxidants and medicinal plants has gained enormous popularity and emerged as a potential therapeutic to prevent free radical generated damage in the human body.
May 11, 2021 · Studies have increasingly evidenced the close association of tannins and less-studied compounds with antioxidant activity in medicinal and food plants 12,18,19,26,48. Thus it is expected that ...
Jan 3, 2023 · Tables 1 and 2 provide information on medicinal plants with antioxidant potential. Several reports have linked free radicals to the occurrence of several ailments like diabetes, cancer ...
Jul 1, 2011 · Recent reports indicate that there is an inverse relationship between the dietary intake of antioxidant-rich foods and the incidence of human disease (Sies, 1993).However, synthetic antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), have been widely used as antioxidants in the food industry and may be responsible for liver damage and carcinogenesis (Grice ...
Ÿ Exploring the mechanisms underlying the antioxidant activities of medicinal plants at the molecular level. Ÿ Assessing the potential applications of medicinal plant antioxidants in healthcare and disease prevention. Ÿ Identifying areas for further research and development to enhance our understanding of the antioxidant potential of
Jan 1, 2011 · Antioxidant activities of plant extracts were assayed by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) method, with ascorbic acid as the standard. Data analysis was conducted by two-way ANOVA with ...
Jun 21, 2022 · Medicinal plants are used for health care either directly or indirectly as they are rich source of several ingredients. Ethno-medical traditions prove that they have a great therapeutic value and are important bioresources. Phytochemicals are naturally occurring compounds having immense antioxidant potential and are of great interest in securing health benefits of consumers. Each portion of ...
The biological activity of plant extracts is also associated with antioxidant activity [30,67]. The topical application of antioxidants, including plant extracts, supports the endogenous defence mechanisms of the skin, helping to reduce UVR-mediated oxidative damage and prevent OxS-mediated diseases [ 124 , 125 ].
In ethanopharmacological and nutraceutical investigations, in vitro antioxidant activity assessment methods are often used to screen and confer antioxidant potential to plants or their phytochemicals and sometimes to understand the probable mechanism of action of plant antioxidants 62. In the case of medicinal plants, these assays are used to ...