Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Organ Donation and Transplantation - Current Status and Future Challenges

Special Offer!
Applies to all print copy prices | Offer ends 15 December 2024 Enter the code 20PRINT to unlock the offer

Order this book in hard copy and enjoy a 20% discount! Use the promo code 20PRINT during the ordering process.
And that's not all! You'll also receive a complimentary bag with an inspiring quote.
Code copied to clipboard!
Valid Until: 15 December 2024
Note: All orders include FREE SHIPPING
(DAP Incoterms – Import duties and taxes are not included).
If you require assistance, contact us at [email protected].
Organ Donation and Transplantation: “Life after Death”
Submitted: 13 November 2017 Reviewed: 03 April 2018 Published: 25 July 2018
DOI: 10.5772/intechopen.76962
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Organ Donation and Transplantation - Current Status and Future Challenges
Edited by Georgios Tsoulfas
Chapter metrics overview
2,233 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Organ donation is defined as giving an organ or part of an organ to be transplanted into another person. Organ transplantation is the only option to save lives in patients affected by terminal organ failures and improve their quality of life. However, there is a disparity exists between the supply and demand of donated organs, leads to a loss of many lives. The number of organ transplantation have gradually increased in the last two decades and provide excellent results in children and young adults, and are challenging by the growing proportion of elderly transplant patients with co morbidity. The results of organ transplantation continue to improve, as a consequence of the innovations and the improvements in peri-operative management. This chapter describes organ donation and transplantation and its trends and challenges.
- organ donation
- psychosocial
Author Information
Kanmani job *.
- Amrita College of Nursing, Amrita Vishwa Vidyapeetham, Kochi, India
Anooja Antony
*Address all correspondence to: [email protected]
1. Introduction
Organ donation is defined as giving an organ or part of an organ to be transplanted into another person. Organ transplantation is the only option to save lives in patients affected by terminal organ failures and improve their quality of life. However, there is a disparity exists between the supply and demand of donated organs, leads to a loss of many lives. The number of organ transplantation have gradually increased in the last two decades and provide excellent results in children and young adults, and are challenging by the growing proportion of elderly transplant patients with co morbidity. The results of organ transplantation continue to improve, as a consequence of the innovations and the improvements in peri-operative management.
Organ transplantation currently depends on the availability of human organs. Their scarcity means that there is a waiting list of almost 63,000 in the European Union, and over 100,000 people in the United States according to the recent survey. The process of obtaining organs for donation and transplantation purely depends on the resources of health services and by health professionals’ performance in potential donor identification and management tasks. However, in accordance with the current legislation it is mainly subjected to a personal or family decision, strongly mediated by psychosocial processes. Therefore, the need to analyze and intervene both in the practices of the professionals involved in the process of organ generation and in the attitudes of the general population need to stressed and addressed [ 1 , 2 , 3 , 4 , 5 ].
2. Organ transplantation and organ donation: an overview
Organ transplantation involves the surgical implantation of an organ or section of an organ into a person whose own organ is failing. The donor organ may come from both deceased individual as well as from a living donor. The patients psychological and behavior aspect as well their emotional response and mental health and adherence to medical regimen should be assessed before and after organ transplantation. The living donor’s psychological response towards organ donation (most commonly for kidney and liver segment transplantation) is an important aspect to consider in the transplantation process.
Organ donation is defined as “giving an organ or part of an organ to be transplanted into another person” (Organ procurement of Transplant Network (OPTN), 2015), organ donation has the potential to save lives. The organs donated from one single donor can save up to eight lives. Organ transplantation may be one of the options left to sustain someone’s life. However, the disparity that exists between the supply and demand of donated organs, leads to a loss of many lives. Based on recent OPTN data, approximately 21 people will die each day while waiting for a transplant in the United States (US). Currently, 123, 358 people are awaiting organs and on the transplant list in the US with this number growing and the number of donated organs declining.
Asian Indians are more likely to have higher rates of having obesity and diabetes when compared with other Asian subgroups which make them at an increased risk of needing a donated organ [ 35 ]. These conditions can lead one to develop coronary artery disease and hypertension which then can lead to chronic kidney disease and other chronic illnesses. Patients who suffer from chronic kidney disease need regular dialysis which can ultimately lead them to organ transplantation to improve one’s quality of life. Also, conditions such as diabetes and obesity can be detrimental to one’s life and can lead to fatty liver disease which can lead to chronic liver disease requiring liver transplantation if the liver decompensates.
The development of organ transplantation in the second half of the 20th century has been a remarkable achievement. Recently; organ transplantation is one of the most effective options for those with an end-stage organ failure. Its success has been basically dependent on public awareness, support and active participation. Without these factors, the efficiency of organ transplantation and the consequent saving or extension of lives would have undoubtedly suffered adversely.
The number of patients in need of organ transplantation has increased at a rapid pace; in contrast, the number of available organs has increased only slightly. Expanded criteria for donor selection, such as older age, have resulted in more people who meet the criteria for brain death becoming organ donors although fewer organs are transplanted from each donor. Improvements in automobile and highway safety, as well as increased enforcement of gun control laws, have also contributed to a plateau in the number of young, healthy donors. Public education efforts that encourage organ donation may be effective in getting more people to sign organ donor cards, but most individuals who do so will never be in a position to become organ donors.
Faced with increasing numbers of patients who need transplantation, deaths on the waiting list, and a fixed number of available organs, some transplant programs are working to increase the number of transplants from living donors. Although living donation has always been an option for some types of transplants, many programs have been reluctant to promote it, as living donation requires invasive surgery on a healthy person with associated risks of morbidity and mortality. For example, since dialysis is an option for patients with end-stage renal disease, surgery on a healthy donor may be difficult to justify, despite the dialysis patient’s diminished quality of life.
The most important in organ donation is to maximize the psychological status and well-being of the donors before and after transplantation has become the foremost goal of all transplantation centres. The psychological issues that mainly concern with the living organ donation includes prevention of psychological harm, ensuring the donors are fully informed and decide to donate without coercion, monitoring donor psychosocial outcomes are intimately linked to the factors that historically served as barriers to use of organs from living donors. These barriers can be overcome by the motivating of the public and creating awareness and responsibility among oneself.
Organs that can be transplanted from the living donor includes one kidney, part of intestine, pancreas, islets of Langerhans, bone, part of liver, one testis, bone marrow and blood. The organ that can be transplanted from the deceased donor are heart, kidney, pancreas, stomach, hand, skin, blood vessels, lungs, liver, intestine, testis, cornea and heart valve.
Autograft: Transplanting a person’s tissues from one site and use it in another site of his body and is called autograft. For example, removal of skin from the legs and using it for damaged skin face or other exposed part.
Allograft: Transplant of an organ between two genetically non identical individuals, it is called allograft. Due to the genetic difference, the donor’s organ will be treated as foreign by the recipient and will try to destroy it. This is called s rejection.
Isograft: Transplant of organ/tissue from a donor to genetically identical recipient is called isograft. There will not be any immune response hence no transplant rejection.
Xenograft: Transplantation of organ/tissues forms one species to another species. For example, the heart valve of pig is transplanted successfully to human.
Split transplant: An organ like liver retrieved from the deceased donor can be divided between two recipients, usually an adult and a child.
Domino transplant: When the lungs are to be transplanted, surgically it is easier to replace them along with the heart. If the recipient’s original heart is healthy, it can be transplanted into another recipient in the need of one.
ABO incompatible transplantation: The immune system of young children aged below 12 months might have developed fully. They can receive organs from incompatible donors.
Live donors: A living person, mentally and physically healthy can donate one of a paired organ, part of an organ or a tissue. The organs donated are kidneys, part of live, one of the lung, part of small intestine, skin, bone marrow, one of the testis and one of the ovaries. Live donor can either be related or unrelated.
Unrelated donors: For altruistic reasons, a person can donate one of his organs to an unrelated donor. According to TOHO act, the unrelated donor should be known to the recipient and have some obligation to him. It has to be established that there is no monetary transaction between them. But in many other countries, even a stranger can donate one of his organs to a needy person on altruistic grounds.
Deceased donors: Organs are harvested from brain dead person whose respiration and circulation are maintained artificially. Brain dead has to be certified by a team of doctors nominated by Government I every organ retrieval centers.
Paired exchange: When a living donor is not compatible with the related recipient, but may be compatible for another recipient. That second recipient related donor is compatible to the first recipient, then permission can be granted for transplantation. The surgery for all four donors and recipient are conducted simultaneously and anonymity is kept until after the transplant.
Spousal donation: A spouse can donate an organ to the partner. It has to be recorded that the couple is legally married.
3. Current scenario: trends

Despite advances in medicine and technology, and increased awareness of organ donation and transplantation, the gap between supply and demand continues to widen. Each year, the number of people in the waiting list is increasing in both donor and transplant. The donation statistics according to OPTN Annual report shows that in 2016, total of 41,335 organs were donated. It can be either deceased or living and four out of five donations came from deceased donors and four out of ten from living donors. According to the report by OTPN 2018, 115,033 people need life-saving organ transplant, of those 74,926 people are the active waiting list candidates.
The real reason behind a living person’s interest in donating one’s organ is important to determine but it is often difficult. Now days, money has become the motivation for donation. The relationships also have played a great role in increasing donation rates. The shortage of available organs can be reduced if; people choose to donate their organs after they die. If more people did that the issue regarding organ shortage can be minimized.
The trend is expected to accelerate each year. Many organ procurement and the Joint Commission on Accreditation of Healthcare Organizations actively participate to increase the donation rates. The organizations take various to steps against traditional social taboos.
The approach, known as “donation after cardiac death” (DCD), usually involves patients who have suffered brain damage, such as from a car accident or a stroke. After family members have made the difficult decision to discontinue a ventilator or other life-sustaining treatment, organ-bank representatives talk to them about donation. Sometimes, the donor is suffering from an incurable disease also end up with the decision of organ donation.
According to U.S Department of Health and Human Services, more than 1,23,000 men, women and children currently needed life-saving organ transplants every 10 minutes and another name is added to the national organ transplant waiting list. In 2014, more than 8500 deceased donors made possible approximately 24,000 organ transplants. In addition, there were nearly 6000 transplants from living donors. In India, nationally with a population of 1.2 billion people, the statistics stands 0.08 persons as organ donor populations. Mrithasanjeevani, Kerala network of organ sharing which began in 2012, also states that the need for organ transplantation is high as the patients in waiting list is increasing day by day who requires organ transplantation.
The need for organ has gone up substantially all over the world. India also suffers from acute organ shortage with little to no solution for this issue. It is estimated that every year 1.5 lakh people suffer from renal failure out of which only 3000 people get donors. Similarly, every year around 2 lakh people die of liver failure or cancer and rarely get any help in the form of organ donors. It is the same for heart patients, for every 50,000 heart attack patients there are only 15 hearts available for transplant. Therefore, there is an urgent need for widespread campaigns to spread awareness about organ donation in India and to bridge the gap between supply and demand. The numbers that are mentioned here are estimates and real numbers could be far more than this, it is scary because this means very few people get relief and get a second chance in life.
The main reasons for organ shortage in India are mainly ignorance and lack of knowledge. People are not well informed enough about the benefits of organ donation. Today social media and so many other forums can promote the positives of organ donation and how it will save so many lives if more people register themselves for organ donation. The reason for organ shortage is myth and superstition. Many people do not want to donate their organs even after death because of so many myths and superstition they are instilled with. People with existing medical condition or old people, who wish to donate, do not donate thinking they are not fit or eligible. Almost everyone can donate some part or the other unless you have any extreme medical condition.
The need for organ donation is necessary because out of the 1.5 lakh people who need kidney in India only 3000 people receive them, only 1 out of 30 people receive kidney and 90% of people in the waiting list die without getting any donor. Around 70% liver transplants are dependent on a live donor but 30% dependent on cadaver (corpse) donations. Hence, there is an urgent need to increase the organ donation rates and give a person a second chance in their life.
4. Challenges in organ donation
As far as the challenges concerned it includes mainly donor’s motives for donation, the predominant ways in which donors arrive at the decision to donate, and the donors’ psychological status and its relationship to their fitness as donors.
4.1. Pre-donation challenges
4.1.1. donor’s motives.
Most donors are likely to be motivated by multiple factors. These factors include intrinsic factors (e.g., desires to relieve the suffering of another or to act in according to the religious convictions) and extrinsic factors (e.g., the social pressures or perceived norms) that may operate simultaneously. The particular combination of motivational forces will also differ depending on whether and how the donor is related to the recipient.
Among living related donors, it has long been assumed that family members or emotional partners are motivated primarily for saving the lives of their loved ones. Such motives are indeed the most commonly expressed feelings, as noted in a variety of studies over the past 30 years. Among nondirected living donors (individuals donating to unrelated patients whom the donors did not select)(NDLDs), it was identified as the altruistic/humanitarian motives, along with beliefs that the donor’s self-worth would be improved, and feelings of moral and religious obligation or self- identity.
4.1.2. Donor’s decision-making
The motivation for the organ donation is purely on the donor’s decision of organ donation and it may be influenced by many factors including the relationship to the recipients. Decision-making swiftness may indicate the type of decision being made. There appear to be two decision-making approaches that include the moral decision making and the rational decision making. “Moral decision-making” involves awareness that one’s actions can affect another; ascription of responsibility to oneself; acceptance of the social/moral norm governing the behavior; and taking action consistent with that norm. Because moral decision-making does not involve the costs and benefits of a given behavior but, instead, is based on perceived norms governing that behavior, it is likely to lead to non- deliberative, instantaneous decisions. In contrast, “rational” decision-making includes various steps that focus on gathering relevant information, evaluating alternatives, selecting an alternative, and implementing the decision.
4.1.3. Support
It includes mainly the assessment of the donor’s available physical, financial and emotional support. It is necessary to identify whether the donor have someone to provide care in the recovery period, have sufficient financial support and so on. This important to avoid distress if the donor develops any complications. Finally, does the donor have the support of significant others for being a donor, or is he or she choosing to donate over the objections of persons who have a legitimate interest in the outcome of an autonomous decision.
4.1.4. Family attitudes toward donation
Spouse and family attitudes about donation should also be explored. Collateral interviews with significant others is necessary, especially those who will be providing tangible support to the donor during the recovery period, should be conducted whenever possible. Conflicts between potential donors and significant others should be addressed and, ideally, resolved prior to surgery itself in order to avoid conflicts later. Family members should provide a good understanding of the donor’s wishes and motives, even if they agree to disagree to the donor’s decision.
4.1.5. Behavioral and psychological health
The behavioral and psychological health of the donor should also be considered before donation. It is important to identify donor’s lifestyle is sufficiently healthy to reduce unnecessary risk for both donor and recipient. Many potential donors may have some unhealthy behaviors, such as moderate obesity or smoking. It is necessary to identify that there is sufficient time for the donor to reduce risks (e.g., lose weight, stop smoking). Moreover, it needs to be taken care of that the donor is emotionally stable to cope with stresses which may come up before, during, and after the donation. Hence it is important to identify psychological and behavioral status of the donor or else it may affect the quality of life.
4.1.6. Donor-recipient relationship
The relationship between the donor and recipient is a complex matter. Even when both parties are agree for donation and transplant, family dynamics may be complicated, and other family members may assertively involve themselves in the decision-making process. The donor may have unrealizable expectations that transplant will alter his or her relationship with the recipient. The health care team should not expect an ideal relationship in which all interactions between donor and recipient are harmonious. However, obvious tensions and overt psychological issues should be addressed. Joint interviews, involving both donor and recipient, should be avoided early in the evaluation process in order to preserve privacy and give the potential donor the opportunity to express reservations or “opt out” gracefully.
4.1.7. Diversity issues
Non directed donors may have diversity concerns that may affect the organ donation. The potential donors should be assessed for comfort with donation to recipients of different genders, races, religions, sexual orientations, nationalities, ages, underlying diseases, and lifestyles. Donors who express objections, fears, or concerns about who might receive their organ may need to be deferred until they can receive counseling.
4.1.8. Psychological status of potential donors
The potential donor’s psychological status is of greatest concern for donation and transplantation. Concerns have been particularly high in case of unrelated donation (either directed to a specific patient, or NDLD): the willingness or desire to donate to a stranger has been historically viewed with suspicion and as likely to reflect significant psychopathology. There is no doubt that some potential donors will be psychologically poor candidates to serve as donors.
4.1.9. Post-donation challenges
The donors’ perceptions of their physical functional, psychological, and social well-being were found to be either nonsignificantly different from or significantly better than levels reported in the general population. The post challenges mainly includes recipient death or graft loss, donor medical complications, donor history of mood or other psychiatric problems, and poor donor relationships with recipient or family. The other factor is that it may affect the donor’s quality of life if any complication arises.
The post transplantation challenges are many which include minimizing rejection risks, immunosuppression, organ shortage, handling of the stressors of transplantation, psychosocial adaptation and psychological disorders and so on.
4.1.10. Minimizing rejection risks
The twin conditions of antibody sensitization and antibody-mediated rejection remain challenging and frustrating to treat. The recent drugs which are used to desensitize patients or reverse antibody-mediated rejection, especially chronic antibody mediated rejection is totally unsatisfactory. Development of therapies those are more effective and less toxic should be made available. Recent regimens used for antibody desensitization and reversal of antibody-mediated rejection include plasmapheresis, immunoglobulin (IVIG), and rituximab, an anti-chimeric, anti-CD20 antibody. Recently, the proteasome inhibitor Velcade has also been reported to reverse refractory antibody rejection. Eculizumab, a humanized anti-C5 monoclonal antibody appears to protect the renal allograft despite the presence of donor-specific antibodies (DSA). None of these agents have been tested in rigorous studies.
4.1.11. Immunosuppression
This is one of the major challenges after organ transplantation. Many studies have suggested that most of the late graft loss occurs because of immunologic reasons, frequently antibody-mediated. So the approach of minimizing immunosuppression is necessary with the present drugs to reduce toxicities may actually be helpful in the long-term survival of the graft. The toxicities are minimized by allowing more grafts to be rejected by immune mechanisms. Hence, development of effective agents that lack long-term toxicities so that we can maintain optimum immunosuppression over the long-term.
4.1.12. Stressors after transplantation
In the perioperative period, the focus is on the patient’s physical recovery, with possible rejection episodes and other medical complications causing anxiety and emotional strain. Within the first days after transplantation, a postoperative delirium can occur. The patient can present with symptoms of mental confusion, language disturbances, and occasional hallucinations and delusions are often a frightening experience to patients and their families. Acute brain dysfunction can occur in intensive care patients and patients after surgery. The corticosteroids which are administered for immunosuppression cause these problems. Some of the patients experience problems in accepting the new organ from another individual and suffer with feeling of guilt towards the donor which, in turn, can increase psychological stress and nonadherence [ 6 , 7 , 8 , 9 , 10 , 11 ].
In the long-term postoperative period, medication side effects and associated comorbidities become central stressors impeding patient’s life quality. Most common comorbidities seen are infections, diabetes mellitus, hypertension, lipometabolic disorders, adipositas, cardiovascular diseases, oncological diseases, osteoporosis, and chronic kidney failure [ 12 , 13 ]. Furthermore, psychiatric symptoms (e.g., depression, anxiety, agitation, psychosis) and neurological symptoms (e.g., sleep disturbances, cognitive impairment, delirium) can occur as neurotoxic side effects in patients receiving immunosuppressive drugs.
Faced with the multiple health risks, patients often continue to experience anxiety and worries regarding possible retransplantation, serious comorbidities, and death. Even patients in good physical health are confronted with severe challenges, for example, regaining their previously lost or restricted social roles as family members and partners (including sexual activity) and returning to work or taking up other meaningful activities. Financial constraints and legal disputes with health or pension insurance agencies constitute other possible sources of psychological strain.
5. Psychosocial adaptation and psychological disorders
After the transplantation, the psychosocial burden more severe in preoperative period than postoperative period. Nevertheless, patients themselves have to demonstrate considerable coping skills. In the best case, transplant patients learn to adapt to their new situation, often by reevaluating life goals and by focusing on more positive consequences, for example, personal growth. On the other hand, unsuccessful readjustment can lower the quality of life and psychiatric morbidity. The most common psychological disorders among patients before and after transplantation are affective and anxiety disorders.
The literature review shows that prevalence of depression in 20–25% of cases before and after kidney transplantation. Less information is available concerning patients receiving other organs. Prior to and following lung transplantation, depression seems to be prevalent in approximately 30% of patients. Hence these show that the depression is a major challenge after transplantation. These issues can be reduced by personal and social resources (resilience factors), that is, favorable coping skills, self-efficacy, sense of coherence, optimism, and social support.
6. Factors affecting donor’s motivation
There are many factors affecting donor’s motivation which includes feelings of love and responsibility, spiritual motives, and greater success rate of organ donation.
6.1. Feelings of love and responsibility
Motives for donating organ to their relative patients were that they tended to do something for their loved ones. In fact, they feel responsible for their problems. They do not treat others’ problems with indifference and attempted to do whatever they could for resolving the problems experienced by transplant recipients. It is considered as their own responsibilities to help them to get rid of their problems. The feel like they are the ones who need to support their patients.
6.2. Close and constant companionship
Another factor affecting the participants’ feeling of responsibility for donation to their family members was close and constant companionship with recipients. This close and constant companionship made the participants to clearly understand the recipients’ conditions and hence, it had resulted in their decision on organ donation in order to alleviate recipients’ problems. This close and constant companionship with patients help family members understand patients’ problems well and increase their degree of commitment to do something for patient’s pain and discomfort. They also noted that this had made them experience deeper shared emotions with their patients and hence, required them to feel responsible for minimizing their patient’s problems.
6.3. Inability to tolerate recipient’s discomfort
Another motive for organ donation was one’s difficulty in tolerating recipient’s discomfort. Love for their sick family members had made the participants feel responsible and decide on doing something for solving their patient’s problems. Their patient’s pain, suffering and discomfort cause a great inconvenience and irritation which lead them to the decision of organ donation. They hoped that organ donation alleviate their patient’s problems [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 36 ].
6.4. Spiritual motives for donation
Religious beliefs played a significant role in motivating to organ donation. Some of them believed that donation was a way for expiating their past sins. They referred to faith in God, reliance on Him, and hope for a successful transplant as the important motives for organ donation. Some of them even accused themselves of causing their family members to develop organ failure and believed that donation was a way for alleviating their feelings of guilt. Such a practice was particularly common among the parents of sick children. Some of them considered donation as a God-approved practice, and noted that God has helped them donate their organs. They noted that they donated their organs for gratifying God and believed that he sees it and help them in all bad situations.
6.5. Greater success rate of organ transplantation
The category is the greater success of organ transplantation. In other words, obtaining information and realizing the greater benefits of organ transplantation had motivated the participants to opt for organ donation. Some of them reported that they had never thought about donation until obtaining information from their patient’s physicians. However, after obtaining adequate information, they had made an irreversible decision about organ donation. Accordingly, a major motive for organ donation was the lower likelihood of organ rejection.
7. Measures to overcome challenges for organ donation
The decisions regarding organ donation based on the personal beliefs (religious, cultural, family, social and body integrity) levels of knowledge about organ donation and previous interaction with the health care team. Many maintained positive attitudes to organ donation despite significant reservations about the organ donation process. Resistance to organ donation found to be less in the case of living donation for family.
There are some religious beliefs that can have both positive and negative influences, these often stemmed from uncertainty or misrepresentation of religious edicts. One solution would be to actively engage religious leaders in the transplant community, especially when it has been reported that, across the major religions, there are very few cases where organ donation can be seen to be inconsistent with religious beliefs. Religious leaders should be made available in hospitals and other transplantation setting to assist families in making decisions regarding organ donation and potentially to remove the misperceptions. Staff members who are involved in approaching families to request consent for donation should be part of the awareness programs and resources about religious concerns. Similarly, cultural sensitivity to issues such as apprehensiveness to discuss death among certain groups or individuals and the importance to many of death rituals may improve dialog regarding organ donation.
Studies have shown that engaging some minority groups in the health care system and creating a sense of belonging and ownership can improve compliance with organ donation. As a consequence, more efforts should be made to create positive interactions within the health care team members, especially for minority groups, to improve the organ donation rates. Although many of the studies have showed that higher socio-economic status and education were associated with a stronger willingness to be an organ donor. Some of the strong reservations held, even among those with generally positive views towards donation, such as concerns that agreeing to donation would discourage doctors from caring so much about saving their lives in case of an emergency or that it would result in the premature removal of their organs or indeed prevent them from having an open coffin at their funerals, are examples of very real barriers that can be readily addressed through information. Through a proper awareness and motivation the donation rates can be improved which can save many lives.
7.1. Psychological care
Psychological consultation is essential for all disease stages enabling patients to better cope with their extraordinarily stressful situation. A need for psychological care was found in up to 50% of transplant patients. Educational and supportive therapies are of utmost importance but also cognitive-behavioral interventions including relaxation techniques can also be considered. Less common methods like hypnotherapy and “Quality of Life Therapy” have also been utilized for overcoming the challenges.
Moreover, family members as well as caregivers of transplant patients show increased psychological strain before and after transplantation. Family counseling, and psychotherapeutic support, can help reduce psychological strain, thus also maintaining the valuable social support provided by care givers and family members of the transplant patient. Henceforth, the family and care givers should also be considered in psychosocial evaluation to overcome the problems.
7.2. Alternative methods to increase donation
In view of ethical, legal and political issues, it was deemed important to obtain some opinion about alternative methods to increase organ donation rates. Financial incentives were given to increase organ donation. Many in both donor and non-donor groups were given a reasonable incentive. Education and dissemination of information about donation and transplantation was important to increase organ donation rates. There was nearly universal agreement that implied consent (presumed consent) should not be tried. The use of financial incentives was not markedly opposed (some accepted the idea of funeral expense reimbursement), although there was not strong support either. In general, methods to increase organ donation had not been well thought out by either donors or nondonors indicating, perhaps, that the assumption of altruism or motivation is the best way to increase the donation rates.
8. Responsibilities of nurses in organ donation and transplantation
Organ and tissue transplant nurses need comprehensive and scientific knowledge. They include the evaluation and management of deceased donors, transplant recipients, potential donors or live donors, teaching and counseling of transplant recipients and live donors related to self-care management, healthy life and a peaceful death when this is imminent. This is important in order to improve the posttransplant quality of life.
Nurses have important role in the development of a successful transplantation program. They are key members of the team that works to deliver care to patients and relatives, through the use of technological, logistic and human resources, with a view to coordination, care, education and research on organ and tissue donation and transplantation. Therefore, the nurses need adequate knowledge on the principles of good ethical principles and should have resources available for them to assess patient’s risks and social issues related to organ transplants and donation. The researchers hope that the future studies will encourage further researches on the role and responsibilities of nurses.
9. Conclusion
The organ donation decision is a complex one, based strongly on personal beliefs. There are some factors, such as religious and cultural beliefs, that are seemingly intractable and are often cited as reasons for a refusal to donate. In this chapter, it is shown that these have often been found to be tied in with more complex issues such as a distrust of the medical system, misunderstandings about religious stances and ignorance about the donation process. Interventions to better engage the community, including disadvantaged and minority groups, to foster trust and provide information represent promising opportunities of promoting organ donation in the future.
Donor motives directly contribute to their decision to donate, is not uniform and is influenced by multiple factors. Majority of the donors were relationship oriented donor, whose major motives were desires to relieve the suffering & save the life of their loving ones. Creating awareness to the organ donation will directly influence the donor motives and willingness. By deriving the motives many more intervention to improve the willingness to be a living organ donor can be evolved. Recruitment of living donors represents a medical and moral responsibility. The possibility of organ removal from healthy donor to a recipient needs great inner motivation. Saving one’s life is divine.
The psycho social assessment must be made as a routine part of the nursing process. These assessments are meant to identify patients at risk for poor outcomes, provide guidelines for their management and improve the post-transplant quality of life [ 6 ]. “Because donated organs are a severely limited resource, the best potential, recipients should be identified. The probability of a good outcome must be highly emphasized to achieve the maximum benefit for all transplants” (OPTN/UNOS Ethics committee General Considerations in Assessment for Transplant Candidacy White paper-2010).
- 1. United Network for Organ Sharing. 1999 Annual Report of the U. S. Scientific Registry of Transplant Recipients and the Organ Procurement and Transplantation Network.: Transplant Data 1989–1998. Richmond, Va: US Department of Health and Human Services, Health Research Services Administration, Office of Sponsored Projects, Division of Transportation, and United Network for Organ Sharing; 2000
- 2. Alexander JW, Zola JC. Expanding the donor pool: Use of marginal donors for solid organ transplantation. Clinical Transplantation. 1996; 10 :1-19
- 3. Kumar A, Mandhani A, Verma BS, et al. Expanding the living related donor pool in renal transplantation: Use of marginal donors. Journal of Urology. 2000a; 163 :33-36
- 4. Mandal AK, Kalligonis AN, Ratner LE. Expanded criteria donors: Attempts to increase the renal donor transplant pool. Advances in Renal Replacement Therapy. 2000a; 7 :117-113
- 5. Simmons RG et al. Gift of Life: The Social and Psychological Impact of Organ Transplantation. NY: Wiley; 1977. Reprinted with additions, Brunswick, NJ: Transaction Books, 1987
- 6. Corley MC et al. Attitude, self-image, and quality of life of living kidney donors. Nephrology Nursing Journal. 2000; 27 (1):43-52
- 7. Goldman LS. Liver transplantation using living donors: Preliminary donor psychiatric outcomes. Psychsomatics. 1993; 34 (3):235-240
- 8. Pradel FG et al. Exploring donors’ and recipients’ attitudes about living donor kidney transplantation. Progress in Transplantation. 2003; 13 (3):203-210
- 9. Fellner CH. Renal transplantation and the living donor. Decision and consequences. Psychother Psychosomat. 1976/77; 27 :139-143
- 10. Toronyi E et al. Attitudes of donors towards organ transplantation in living related kidney transplantations. Transplant International. 1998; 11 (Suppl 1):S481-S483
- 11. Henderson AJZ et al. The living anonymous kidney donor: Lunatic or saint? American Journal of Transplantation. 2003; 3 :203-213
- 12. Jacobs CL et al. Twenty-two nondirected kidney donors: An update on a single center’s experience. American Journal of Transplantation. 2004; 4 :1110-1116
- 13. Matas AJ et al. Nondirected donation of kidneys from living donors. The New England Journal of Medicine. 2000; 343 (6):433-436
- 14. Olbrisch ME et al. Psychological, Social and Behavioral Characteristics of Living Donor Candidates for Adult Liver Transplantation. Biennial Meeting on Psychiatric, Psychosocial and Ethical Issues in Organ Transplantation. Santa Monica, CA; February, 2005
- 15. Crowley-Matoka M et al. Long-term quality of life issues among adult-to-pediatric living donors: A qualitative exploration. American Journal of Transplantation. 2004; 4 :744-750
- 16. Karliova M et al. Living-related liver transplantation from the view of the donor: A 1-year follow-up survey. Transplantation. 2002; 73 (11):1799-1804
- 17. De Graaf Olson W, Bogetti-Dumlao A. Living donors’ perception of their quality of health after donation. Progress in Transplantation. 2001; 11 (2):108-115
- 18. Beavers KL et al. The living donor experience: Donor health assessment and outcomes after living donor liver transplantation. Liver Transplant. 2001; 7 (11):943-947
- 19. Lennerling A et al. Becoming a living kidney donor. Transplantation. 2003; 76 :1243-1247
- 20. Pradel FG. Exploring donor’s and receipient’s attitudes about living door kidney transplantation. Progress in Transplantation. 2003; 13 (3):203-210
- 21. Smith MD. Living related kidney donors. A multicenter study of donor education, socioeconomic adjustment and rehabilitation. American Journal of Kidney Diseases. 1986; 8 (4):223-233
- 22. Jacobs CL. Expanding living donor options. Minnesota Medicine. 2001; 84 :46-48
- 23. Switzer GE. Understaning donor;s motivations. A study of unrelated bone arrow donors. Social Science & Medicine. 1999; 45 (1):137-147
- 24. Rosenthal JT. Expanded criteria for cadaver organ donation in renal transplant. The Urologic Clinics of North America. 1994; 21 :283-292
- 25. Shiel AGR. Transplantation and society. In: Ginns LC, Cosimi A, Morris PJ, editors. Transplantation. Malden, MA: Blackwell Science; 1999. pp. 897-913
- 26. Callender CO, Bay AS, Miles PV, Yeager CL. A national minority organ/tissue transplant education program: The first step in the evolution of a national minority strategy and minority transplant equity in the USA. Transplantation Proceedings. 1995; 27 :1441-1443
- 27. Surman OS et al. Live organ donation: Social context, clinical encounter, and the psychology of communication. Psychosomatics. 2005; 46 :1-6
- 28. Papachristou C et al. Motivation for living-donor liver transplantation from the donor’s perspective: An in-depth qualitative research study. Transplantation. 2004; 78 :1506-1514
- 29. Haljamäe U. Remaining experiences of living kidney donors more than 3 yr after early recipient graft loss. Clinical Transplantation. 2003; 17 :503-510
- 30. Hamburger J, Crosnier J. Moral and ethical problems in transplantation. Rapaport F, Daussert J. Human Transplantation. NY: Grune & Stratton; 1968. pp. 68
- 31. Sadler HH et al. The living, genetically unrelated, kidney donor. Seminars in Psychiatry. 1971; 3 :86-101
- 32. Walter M et al. Psychosocial outcome of living donors after living donor liver transplantation: A pilot study. Clinical Transplantation. 2002; 16 :339-344
- 33. Fukunishi I et al. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001; 42 (4):337-343
- 34. Crowley-Matoka M, Switzer G. Nondirected living donation: A survey of current trends and practices. Transplantation. 2005; 79 (5):515-519
- 35. Boparai J, Davila H, Chandalia M. Cardiovascular disease in Asian Indians living in the United States. Current Cardiovascular Risk Reports. 2011; 5 :240-245
- 36. Ji WR, Frederick B, Hsin CY. Trends in prevalence of Type 2 diabetes in Asians Versus Whites results from the United States National Health Interview Survey 1997-2008. Diabetes Care. Feb 2011; 34 (2):353-357
© 2018 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Organ donation and transplantation.
Published: 25 July 2018
By Young-Nam Roh
1548 downloads
By Bulang He, Xiuwu Han and Michael A. Fink
2616 downloads
By Félix Cantarovich
2029 downloads
IntechOpen Author/Editor? To get your discount, log in .
Discounts available on purchase of multiple copies. View rates
Local taxes (VAT) are calculated in later steps, if applicable.
Support: [email protected]
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
Organ Donation and Transplantation: “Life after Death”

2018, Organ Donation and Transplantation - Current Status and Future Challenges
Organ donation is defined as giving an organ or part of an organ to be transplanted into another person. Organ transplantation is the only option to save lives in patients affected by terminal organ failures and improve their quality of life. However, there is a disparity exists between the supply and demand of donated organs, leads to a loss of many lives. The number of organ transplantation have gradually increased in the last two decades and provide excellent results in children and young adults, and are challenging by the growing proportion of elderly transplant patients with co morbidity. The results of organ transplantation continue to improve, as a consequence of the innovations and the improvements in peri-operative management. This chapter describes organ donation and transplantation and its trends and challenges.
Related papers
INTERNATIONAL JOURNAL OF SCIENTIFIC DEVELOPMENT AND RESEARCH, 2023
Transplanting a healthy organ into a person who lacks that organ can save their life and boost their quality of living. While organ transplants have improved greatly over the past two decades, with generally excellent outcomes for children and young adults, the increased proportion of older transplant patients with comorbidities poses new challenges. Transplants are essential for the treatment of patients with irreversible diseases of the liver, heart, or lungs, and renal transplantation improves patient survival compared to dialysis. There has been a consistent increase in the number of solid organ transplant programmes, but this growth falls well short of meeting worldwide needs. Giving someone a second chance at life is possible only via organ and tissue donation. Giving to charity has far-reaching benefits for society as a whole, not just for the individual or family receiving the donation. In 2021, there were 1,44,302 organ transplants worldwide; India accounted for 8% of that total with its 42,259 transplants. Therefore, decreasing the number of patients in need (the waiting list) of an organ can be accomplished by increasing public awareness of the significance of volunteer enrolling for organ donation. Index Terms-organ donation, tissue donation, body donation, transplantation, organs and tissues of transplantation. I.
Global Journal of Health Science
The majority of countries are battling with a high incidence of organ failure such as the kidneys, heart, lungs, pancreas, and liver. The only solution that can remedy the plight of patients facing the strong likelihood of death as a result of malfunctioning body organs is organ donation and transplantation. The intention of this literature study is to assess progress in organ donation and transplantation. This study has benefitted immeasurably from previous scientific investigations. Four hundred and thirty-one published papers were selected from different accredited journals. The study found that many of the countries that have implemented the opt-in system are struggling to close the gap between the high demand for and the actual availability of life-saving organs due to low rates of registered and committed organ donors. The majority of patients that are contending with end-stage diseases are added to the organ donation waiting lists, but have little hope of receiving life-prolo...
BMC Anesthesiology
Background Organ transplantation (OT) is one of most successful advances in modern medicine. For patients with end stage disease, transplantation most often provides their only chance for survival. Even before the first transplant was performed, it was clear that OT could only be successful with a multidisciplinary approach. The history of OT has involved a series of breakthroughs in medicine that has influenced all aspects of health care. As you will see, for nearly a century, the contributions of specialists in anesthesiology and critical were largely underrepresented in the worlds literature. Short history of organ transplantation The earliest descriptions of OT can be found in ancient Greek, Rome, Chinese, and Indian mythology involving bone, skin, teeth, extremity, and heart transplantation [1, 2]. In the sixteenth century, Italian surgeon Gasparo Tagliacozzi used skin transplant for plastic reconstruction. He was the first to describe what we now know is an immunologic reaction when the graft is obtained from a different person. It was only at the end of nineteenth century that OT research began to be both more systematical and better documented. The first animal models (usually dogs) were developed at this time. Early in the twentieth century, French surgeon Alexis Carrel (who later move to the US) developed a new method for vascular anastomoses. Dr. Carrel performed several successful kidney transplants in dogs, developed an approach for vessel reconstruction, and began the practice of cold graft preservation. In 1912 Dr. Alexis Carrel was awarded the Nobel Prize in Physiology or Medicine for his pioneering work [3]. The first human to human transplant was performed in 1933 in the Soviet Union by the Ukrainian surgeon U.U. Voronoy. The blood group mismatched graft was obtained six hours after the donor's death and although the patient survived two days, the graft never produced urine [3, 4]. Despite significant
IP Innovative Publication Pvt. Ltd., 2017
“Organ transplant” is not just a term or a medical procedure, but an idea, a startling yet extraordinary discovery of medical science in the 20th century that has undoubtedly saved millions of lives across the world. ‘An organ is a mass of specialized cells and tissues that work together to perform a function in the body’. So what exactly is organ transplantation? “An organ transplantation is a surgical procedure wherein failed or damaged organ in the human body is removed and replaced with a new one.” The donor and recipient may be at the same location, or organs may be transported from a donor site to another location. Organs and/or tissues that are transplanted within the same person's body are called autografts. Tissues transplanted between members of same species are called allografts, which can either be from a living or cadaveric source
Intensive Care Medicine, 2013
Asian Journal of Pharmacy and Pharmacology, 2020
Organ transplantation is a medical procedure in which an organ is removed from one body and replaced in the body of a patient who has damaged or missing organs. The person who donates organ is called donor whereas a patient who receives it, is called the recipient. The donor and recipient may be available in the same location, or organs may be transferred from a donor site to the recipient site by a suitable method. Successful transplanted organs to date include the lungs, intestine, uterus, heart, pancreas, liver and thymus and kidneys. Tissues that are transplanted include tendons, skin, bones, valves, cornea, nerves and veins. Worldwide, the most commonly transplanted organs are kidneys, then liver and then the heart. Organ donors are living, or brain dead. Cadaver transplantation is the most challenging, complex and risky areas in the field of medical and surgical science. Overall transplantation requires patience, financial planning, ethical consideration, dedication and expertise of the medical team. It involves major risk of patient's life. But it is considered the most miraculous treatment of the century in the field of medical science. The present review is therefore planned to focus on its importance, types, history, need, process, medications and risks of organ transplantation.
Rambam Maimonides medical journal, 2011
Organ transplantation has progressed tremendously with improvements in surgical methods, organ preservation, and pharmaco-immunologic therapies and has become a critical pathway in the management of severe organ failure worldwide. The major sources of organs are deceased donors after brain death; however, a substantial number of organs come from live donations, and a significant number can also be obtained from non-heart-beating donors. Yet, despite progress in medical, pharmacologic, and surgical techniques, the shortage of organs is a worldwide problem that needs to be addressed internationally at the highest possible levels. This particular field involves medical ethics, religion, and society behavior and beliefs. Some of the critical ethical issues that require aggressive interference are organ trafficking, payments for organs, and the delicate balance in live donations between the benefit to the recipient and the possible harm to the donor and others. A major issue in organ tra...
Current problems in surgery, 2003
O rgan transplantation is currently the standard therapy for end organ failure in those patients who are suitable medically for transplantation. At present, there are more than 80,000 patients who are listed for transplantation in the United States (Table 1). 1 The United States organ transplant waiting list grows an average of 16% to 20% per year. In the past 10 years, the number of registrants has increased from 23,901 registrants to more than 80,000 registrants. In contrast, the number of cadaveric organ donors that are available increased from 4526 donors in 1991 to 5985 donors in 2001 (Fig 1). 2 The number of transplantations that were performed in the United States has also increased from 12,626 transplantations in 1988 to more than 22,000 transplantations in 2000, with the significant increase being related to broader use of living donors and expanded acceptable criteria for cadaveric donors (Fig 2). 2 Of those registrants who are listed for life-saving organs (such as heart, lung, and liver), the 1-year mortality rate while awaiting a transplant ranges from 10% to 25%. The overall mortality rate for all patients on the waiting list is 7.5% and continues to grow yearly. 1 Patients who have irreversible loss of all brain function, but who are maintained on ventilators (ie, brain dead) are the single largest source of transplantable organs. It has been estimated that between 6900 and 10,700 potential donors are available annually in the United States (28.5 to 43.7 donors per million population). Unfortunately, organ procurement efforts are between 37% and 59% efficient in terms of the actual recovery of organs. Efficiency varies greatly by state and organ procurement organization. 3 The most realistic estimates of donor supply place the number of potential donors between 43 and 55 per million population. The range of potential donors is therefore somewhere between 7088 and 25,865. Realistically, estimates must be adjusted on the basis of public attitudes toward donation. Between 53% and 68% of the public is willing to donate the organs of relatives under appropriate circumstances. Subsequently, between 5700 and 9300 donors may be
Canadian Journal of Anesthesia/Journal canadien d'anesthésie, 2006
KIU Journal of Humanities, 2021
This paper aims at an examination of organ transplantation with special focus on the viability or otherwise of transplantation as a lifesaving procedure for patients with end-stage organ failures. It points out that the crucial issues in organ transplantation is centered on question of whether organ transplantation really serves the purpose of saving lives or a death-row and the question of how to ensure justice in the face of organ shortage. This paper therefore argues that organ transplantation which is supposed to serve solely the purpose of life saving is also a death row for those awaiting organs; this is because of the unavailability of organ, unwillingness to donate and the unfavorable role that the criteria for allocation does to others who are not opportune to have the needed organ before death.
Revista de Administração Mackenzie, 2018
Discourse & Society, 2010
Dilemas - Revista de Estudos de Conflito e Controle Social, 2024
CONTEMPORARY CAMBODIAN LABOR AND EMPLOYMENT LAW, 2021
Alteridad, 2024
Clío & Asociados La historia enseñada, 2021
The Journal of Peasant Studies, 2022
perspektiven ds, 2024
International Journal of Computer Applications, 2012
Linear and Multilinear Algebra, 2020
Health, 2022
Medical Journal of Australia, 2014
Jurnal Layanan Masyarakat (Journal of Public Service),, 2022
Diplomacia da saúde e Covid-19: reflexões a meio caminho, 2020
Related topics
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
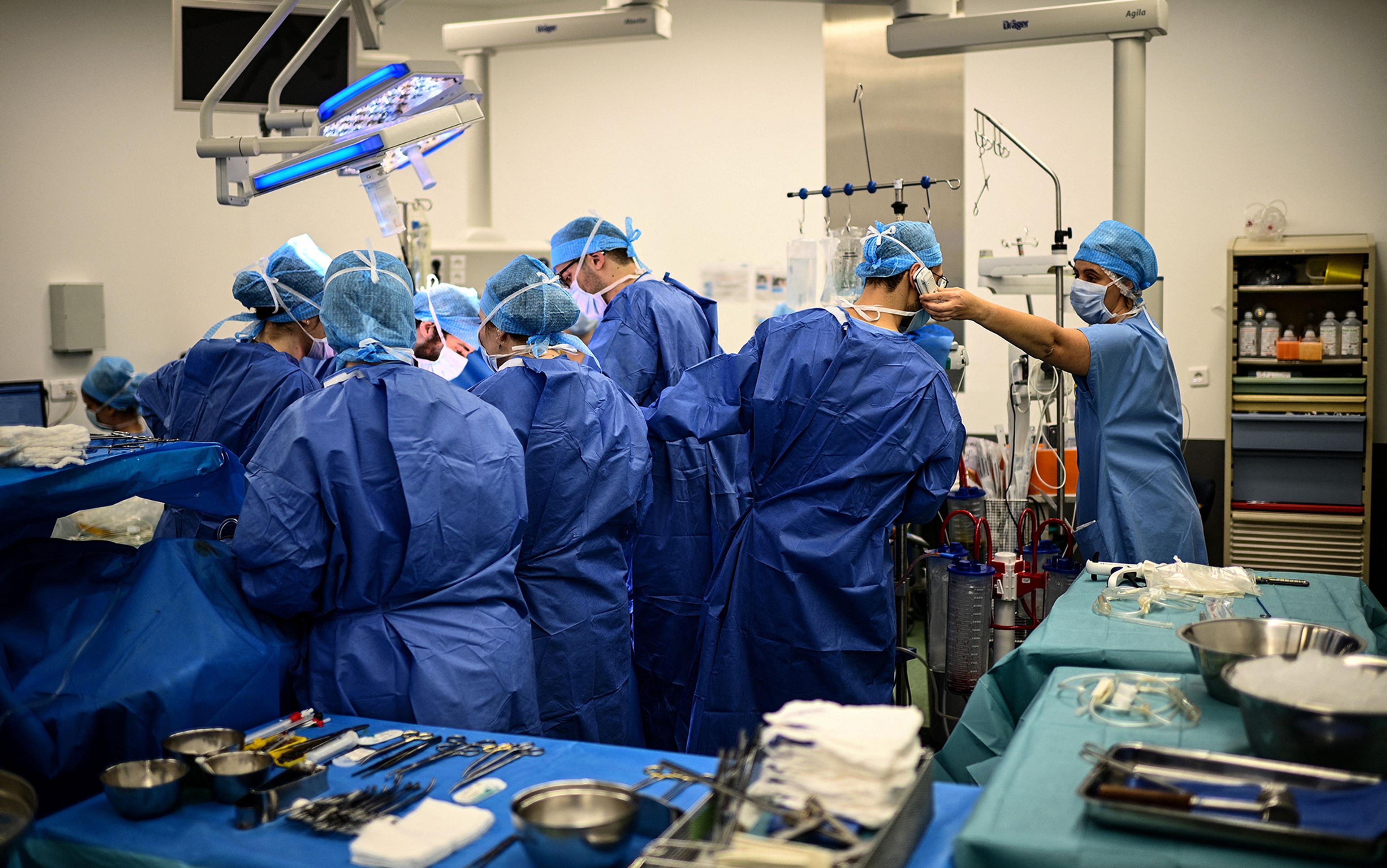
After assessing the lungs of a deceased patient, the thoracic surgeon Thomas Charrier (second from right) gives the green light for transplantation to his colleagues on the phone at the Foch hospital in Suresnes, Paris. This and all photos below taken on 9 December 2022 by Christophe Archambault/AFP. Courtesy Getty Images
Last hours of an organ donor
In the liminal time when the brain is dead but organs are kept alive, there is an urgent tenderness to medical care.
by Ronald W Dworkin + BIO
My patient was dead before I even saw her. She had been in a car accident. Now she was scheduled for organ donation.
She was called an ‘ASA 6’. To estimate operative risk, the American Society of Anesthesiologists (ASA) has a classification system built around how sick a patient is, ranging from a healthy ASA 1 to a moribund ASA 5, the latter meaning someone is not expected to live 24 hours. When the system was created in the mid-20th century, a sixth class for dead people seemed pointless. Death was known to the dead only, and life to the living only, and between the two there had been no bridge. When the definition of death changed in the late 1960s, making it possible for a person to be ‘brain dead’ but with organs still very much alive and available for donation, a bridge appeared and a sixth class was created in the early 1980s. Since 1988, when officials started collecting organ transplant data, almost a million organ transplants have been performed in the United States. Most of the organs have come from brain-dead donors. In 2021 alone, the US had almost 10,000 such donors.
When told of my upcoming case, I had mixed feelings. On the one hand, being in perfect health, unaccustomed to suffering and therefore easily disconcerted by the thought of death, I was horrified. My attitude toward death was like that of a young person standing blindfolded and tied to a post, awaiting a volley from a firing squad. The whole concept made my blood run cold. Yet the case also aroused in me a feeling of relief. Simply put, there was no risk of malpractice, as my patient was already dead. Many anaesthesiologists have such self-centred thoughts when taking care of ASA 6 patients.
I n her early 30s, she had a youthful face, without the traces of severe illness that ICU patients typically have. With her hair wrapped inside a bright, polka-dotted kerchief, she gave off an expression of almost pleasant, good-humoured cheeriness.
Who was this young person whose life had been tragically snuffed out? I jabbed into every crack of her medical record to find out. But little had been written down. Both her life and death seemed simple enough to be chronicled in a few lines. Something had happened inside her brain with the car accident, and the end came.
We wheeled her toward the operating room. Before departing, I pulled the sheet over her exposed feet. Why? I had an objection to her being dead, but I had an even more serious objection to her being undignified. With my patient still partly in the world of the living, I wanted to keep a place for her in the part that pretends to be genteel.
Six hours she had been officially dead. Now she had re-entered the world of the living
When we arrived in the operating room, something turned over inside me, sank, and went cold. After all, she was dead. The day before, she was as whole as me – and now look. She would never rise again.
After we moved her from the gurney to the operating table, the doctors and nurses, so used to taking care of living patients, stared at one another stupidly, as if not knowing why they had come together or why they stood around the table. For a brief moment, each one of us likely had the same supernatural vision, how for the past six hours, after being declared brain dead, this woman had lain under the measureless power of death. Six hours she had been officially dead. Six times had the hour hand on the clock moved – and she had lain dead. Now she had re-entered the world of the living. I would support her blood pressure and pulse. I would make her blood bright red with oxygen. Indeed, she might even wake up and look at us, I fantasised. She might be raised from the dead.
In a race against time, Thomas Charrier checks a monitor during the operation to remove the donor’s lungs
Ghoulish thinking, yet I do not write about this case to be ghoulish. Nor am I trying to stake out a new position in the bioethics debate. My purpose is more practical. Today, artificial intelligence (AI) looms over medical practice. Although unlikely to replace doctors completely, AI makes some medical activities especially ripe targets for takeover, including the harvesting of organs from brain-dead donors. And why not? Bedside manner and the common touch cease to be concerns. Using AI machines rather than doctors to harvest organs also promises to save money.
Yet this impersonal, nonhuman method of organ retrieval may discourage people from becoming organ donors , or from letting dead relatives become so, thereby exacerbating the current organ shortage. People will see pictures of organ retrieval being carried on all around by inanimate machinery in a room completely abandoned by human beings. Bodies will be brought in and sent out, while the invisible, sleepless work of the machines goes on. ‘Please, tell me this is not my end,’ people will fret privately. And they will resist consenting to organ donation.
O rgan retrieval can take place at odd hours because the time between retrieval and transplantation must be minimised. A donor heart or lungs can last only four to six hours outside the body. A kidney, liver or pancreas can last a bit longer. Because donation and transplantation must be synched perfectly, surgical teams must be allowed to work at any hour of the day.
Although donors are dead, managing their anaesthesia can be tricky. To keep their organs healthy, their physiology must be carefully attended to, yet brain death affects each organ system differently. High intracranial pressure can lead to an enormous outpouring of adrenaline, which can injure the heart and other organs as circulation fails. Brain death can cause pulmonary oedema (fluid in the lungs), making it hard to oxygenate the blood, thereby damaging the organs by a second route. Brain death also wreaks havoc on the body’s endocrine system, causing vital hormone levels to fall and damaging organs by a third route.
My patient lay stretched out on the table. The room was silent, as I had not yet placed any vital sign monitors on her body. It was a sinister silence. The monitors typically emit sounds that resemble the unconcerned twitter of birds. In an operating room, they symbolise life. Their absence suggests that a patient is not alive. In fact, mine wasn’t.
Nature will never permit anyone to know the exact point where brain death becomes real death
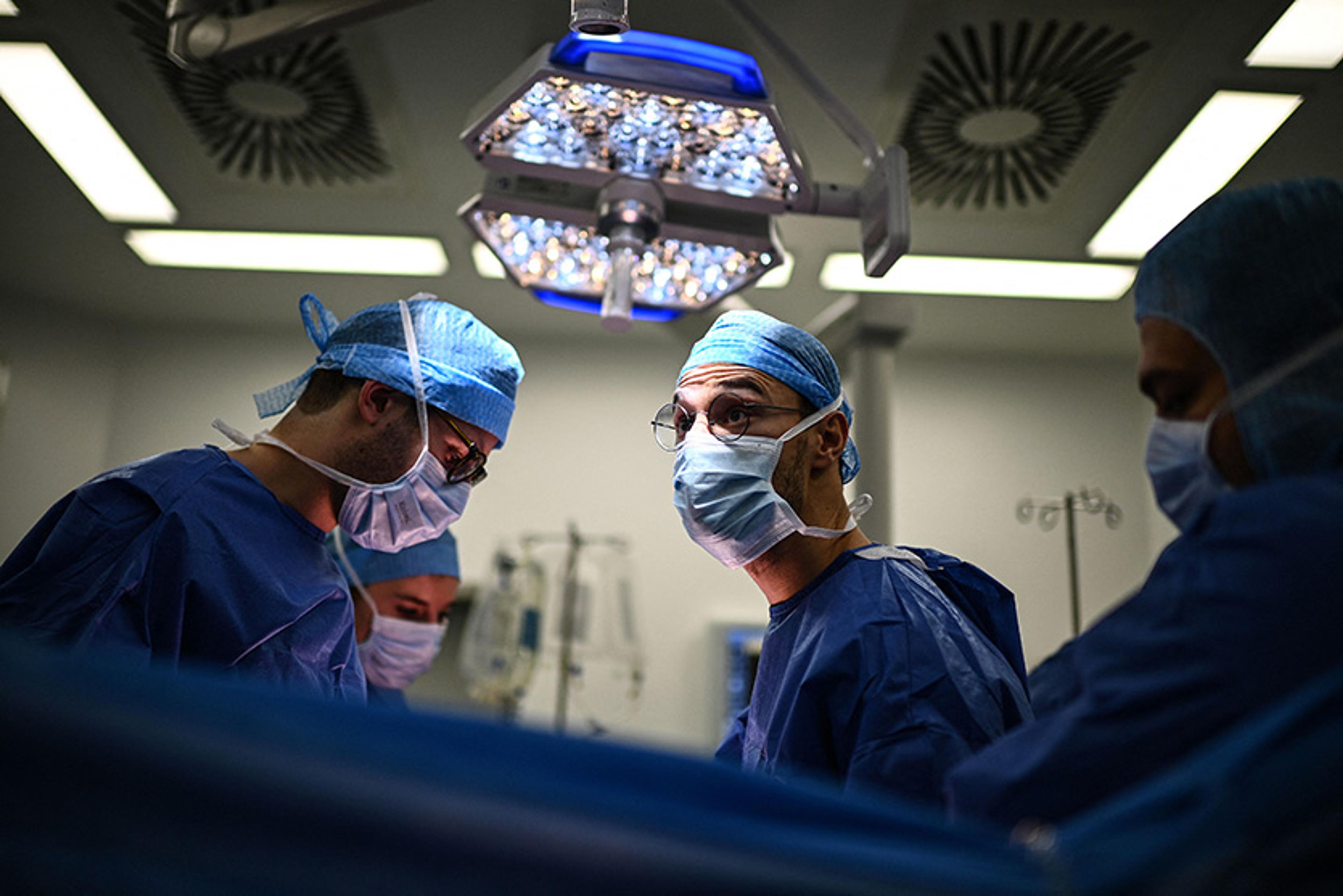
Ready to cut, the surgeon spoke through his headphones to surgeons in other cities waiting for the organs. The rest of us said nothing as he gave them an estimated time of arrival. The team had a real need for a stout word in these last few moments before the operation began. Sensing the mood, the surgeon said some dignified things about how our patient was giving other people a chance to live. Everyone nodded in agreement. He seemed thankful and sincere, yet he had to be that way for, at such a moment, anyone with even a modicum of intelligence would have felt anything else as an affront.
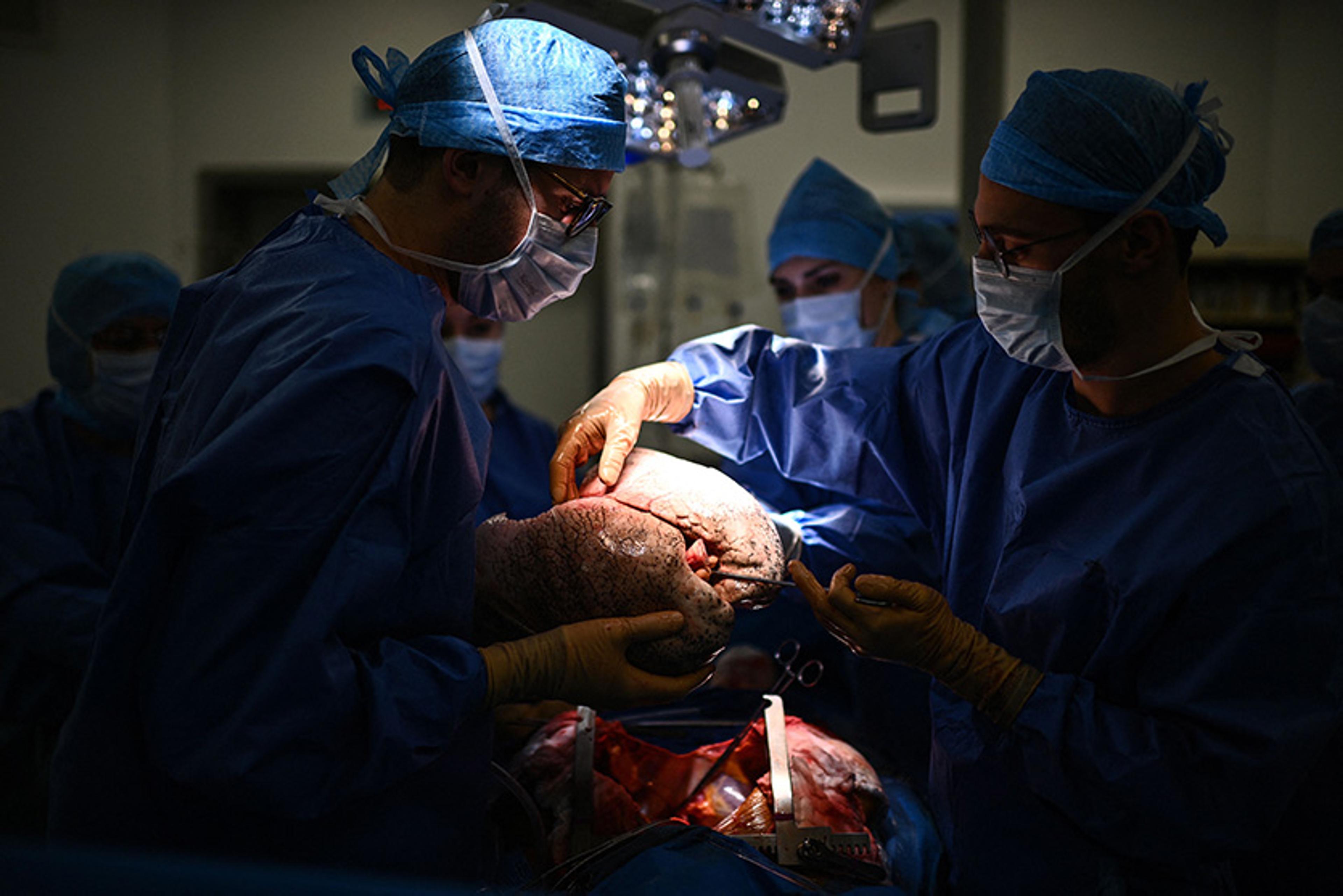
Removal of the donor’s lungs
The surgeon cut into the patient’s chest. Almost immediately the patient’s heart rate and blood pressure jumped. It resembled the powerful surge of life that comes during a period when a person’s very existence and survival are at stake. The jump originated in a spinal reflex that stimulated the woman’s sympathetic nervous system below the level of the brain. Still, it seemed a manifestation of her will to live. Even more so when her hand moved – a sure sign of life! But that, too, was mediated through a spinal reflex.
I gave the patient some anaesthetic gas. I also gave her some opioids. Why the latter? After all, a dead patient doesn’t feel pain. In part because opioids help to lower heart rate and blood pressure directly, but also, I must admit, because I thought my patient might still be a ‘little alive’, whatever that means, and therefore in pain. Irrational on my part, yes, but the secret of life, including the definition of life, still remains the deepest and most mysterious one. Here Nature permits no eavesdroppers; never will she permit anyone to know the exact point where brain death becomes real death. At this, she draws a veil. I wanted to hedge my bets.
The woman’s blood pressure soon dropped too low. I poured fluid into her intravenous line. In the meantime, the surgeon moved hurriedly to extract her heart, clamping the large blood vessels leading both to and from it. Our fast pace betrayed another incongruence. Speed is thought to be a bit unrefined in an operating room. True, it is needed to save money, but ideally the operating room is an ordered world with calm transitions, a world without haste, except during an emergency to save a patient’s life. The visibly hasty fashion in which I and the surgeon worked made it seem like one of those urgent situations. In fact, I was trying to keep her circulation going long enough for the surgeon to tear her heart out.
I transfused a unit of blood, as excessive blood clotting, common after brain death, had made her dangerously anaemic. I turned on the warming mattress lying underneath her to keep her body temperature from dropping below 36 degrees Celsius. Brain death interferes with the body’s ability to regulate temperature, and the resulting hypothermia poses a risk to the organs. Finally, I gave her insulin to control her blood sugar level, as brain death often causes blood sugar to rise. All of these are routine lifesaving measures. In the past, I had used them to fight off death in my sickest patients, but here I had to remind myself that my patient was already dead.
T he surgeon removed her heart. The irrevocable instant had come. It was as when a train starts with a violent jerk, as if to overcome a disinclination to change its state of inertia. For many in the operating room, this was the moment when the woman’s life really ended.
A minute before, I had heard the melodies of the electrocardiogram (EKG) and pulse oximeter without really thinking about them. An anaesthesiologist’s ear is so capable of adapting itself that a continuous din, like the noise of a street or the rushing sound of a river, adjusts itself completely to their consciousness. But the unexpected halt in the sounds startled me into listening – and looking. I stared over the ether screen into the woman’s now-vacant chest cavity. It was shameful and terrible to gaze upon. Part of me felt as if I had abetted a murder around the corner.
I tried to make this last sigh worthy of the moment, a sigh that only another human being could replicate
The surgeon injected the heart with cold preservative and put it in a box. His next target was the lungs. He asked me to manually give the woman one last deep breath so that he could confirm that all parts of her lungs had been expanded before their removal. The breath I gave her was slow and gentle, like a sigh. Indeed, medically speaking, it was a sigh. On ventilators, there is a function labelled ‘sigh’ which, when pressed, gives a patient a single sustained deep breath to open up the lung’s small air sacs. Almost proudly, I imagined the sigh I gave this woman to be more human than what a machine could give. Compressing the anaesthesia bag with my hand, I imagined how she might have sighed in the past, on her own, in the face of some bitter reality, some trick or force of fate, crushing her heart but also uplifting her. I tried to make this last sigh worthy of the moment, a sigh that only another human being could replicate, a deep breath that begins with disappointment, passes into resignation, and ends in acceptance. I crafted that last sigh as if it were the epilogue to a tragedy.
When the woman exhaled her last bit of sigh, I removed her breathing tube. The surgeon took out her lungs and stapled her windpipe shut. At this point, there was little for me to do, and my inactivity plunged me into a sense of nothingness. I felt I was going to gag on my thoughts if I didn’t do something. I walked away from my patient to look inside a cupboard. I opened a few drawers. Then I felt badly for doing so. Although one of us was dead, still there seemed to be two of us here. It’s as if the woman and I were friends and I didn’t want to leave my friend. I went back and stood by her head.
The surgical team removed the rest of her organs and the case finished. Here the woman and I reached a parting of the ways. I stared at her face seriously and fixedly as though I wanted to look my fill and imprint forever on my memory her image. I cannot recall for how long I looked. Great moments are always outside of time.
Our relationship proved significant. Nothing is more characteristic of the total lack of spiritual connection between myself and other patients than the fact that I have forgotten most of their names and faces. But this woman’s name and face I remember. And when I speak of memory, I do not mean something akin to a register kept in a well-ordered office, a place in which documents are laid away in store. I mean something submerged in the rushing stream of my blood, memory as a living organ in which every feeling experienced that day retains its natural essence, its original intensity, its primary historical form.

Charrier and his colleague Ludovic Dupautet en route to hospital with the organ-transport ice chest
M y patient lived longer than what her death certificate says. She lived in my mind during the organ retrieval and continues to live in my memory because I do not want to forget her, and because I cannot forget her. Although I know only her general outlines, our connection satisfies some deep law of harmony underlying all life, in which every person must enter into communion with another person in order fully to live. By that standard, my patient lived past her official time of death.
When AI replaces the anaesthesiologist during organ retrievals, you, the organ donor, will not live any longer than what’s listed on your death certificate. The air inside the operating room is already cold, dry and unpleasant. Various monitors will sit on the top shelf of the anaesthesia machine, regulated by AI. Their special melodic rings will no longer be necessary, as their data will be sent along to AI in silence. Care will be delivered without the mediation of human minds, senses or hands.
Before your lungs are removed, the machine’s ventilator bellows will go up, down, up, down. Capable only of whooshing and not ringing, the bellows will seem to call sadly through the cold air to the monitors sitting above, waiting fruitlessly for a response. What a simple, insignificant movement: up, down, up, down, never getting away from the same place. It will be the only activity surrounding your head amid the metal machines, with their cold, menacing gleam. Nobody will be sitting next to you to wonder about what you were like, or about the things that distinguished your personality and made it special. A vision of the future arises: similarly deserted operating rooms with AI machines all moving automatically, while the people who used to work in them have gone off somewhere to sit dreamily on the grass beneath the sky.
Medicine has tapped a new source of organs in the form of donation after circulatory death
It seems a decidedly unpleasant environment in which to meet one’s end, and enough to discourage those on the fence from becoming potential organ donors. Rather than live a bit longer in the mind of another person, rather than have that person think about you and wonder about you, entertain irrational imaginary concerns about you, slip a little extra narcotic into your intravenous line ‘just in case’, engage in metaphysical speculations about your breathing pattern, and imagine you a friend, you will be alone on the table, and your surgery will more closely resemble a bandit raid. The machines will be determined to take everything, every organ. Reduced to financial terms, it will be as if you were left penniless.
Worse, a new danger will come to overlie the chilling sense of emptiness, scaring even more people away from becoming organ donors. Because there are already too few brain-dead donors – each year, more than 8,000 people in the US die while on the waiting list for organs – medicine has tapped a new source of organs in the form of donation after circulatory death (DCD). These organ donors are not brain dead, although many of them are unconscious. Instead, they lack circulatory or respiratory activity after being disconnected from artificial life-support systems. Without such activity after a few minutes of so-called ‘no touch’, they are declared dead, and their organs become available for donation. Their numbers have been growing over the past two decades. Today, they account for about 10 per cent of transplanted organs in the US.
The problem is that certain medicines necessarily given to these donors before death, but in expectation of death, may hasten their death. The medicines are given for the sole purpose of making their organs more viable for transplantation. Heparin, for example, prevents blood clotting, while phentolamine dilates blood vessels and improves blood flow to the organs. Yet heparin also increases the chances of bleeding into the brain, while phentolamine may lower blood pressure to the degree that a person goes into shock. Although these patients are near death for other reasons, the medicines may become their actual cause of death. This makes doctors uneasy; hence the rule not to give these medicines to donors prone to bleeding or with low blood pressure.
Y et the rule risks driving away potential donors, who will naturally think: ‘Doctors won’t give you these medicines to kill you; on the other hand, they won’t give you these medicines to help you. At some point, doctors aren’t really on your side. But when?’ As the number of brain-dead donors drops, medicine will increasingly rely on DCD donors to compensate. Already sensitised by the image of surrendering their organs to machines in a metallic desert, potential donors will fear being herded into the ‘imminent death’ category to meet some organ quota, a fear only heightened when word gets out that doctors are not always their advocates.
Currently, doctors in the US must get consent before declaring people DCD donors, but another model, called the ‘presumed consent model’, already operates in other countries and allows the recovery of organs without prior authorisation. A cascade of events threatens to turn organ retrieval into a kind of science-fiction nightmare, where organ shortages lead to medicines being given to people to preserve their organs while possibly hastening their death, all without telling them the plan or asking for their permission.
My experience in the operating room serves as a warning to those eager to cut corners and replace doctors with AI machines
Whenever people start to think about becoming an organ donor, they immediately make a leap to their own precious person. Who am I, what am I, what am I without my organs, and so forth. It is part and parcel of being a human being. That some people are willing to surrender their organs after death suggests that, to feel oneself human, they need more than merely having a whole body; they also need an atmosphere of simple humanity. To feel human, people need to feel that they occupy space in the thoughts and feelings of others. It is why they consider becoming organ donors in the first place. They imagine helping others by giving up a part of themselves in the future. In exchange, they imagine the recipients thinking from time to time about what they, their donors, were like. In that way, the donors feel a connection with whomever those recipients might be, and feel somehow fulfilled.

The surgeons arriving back at the Foch hospital with the donor’s lungs
I’d like to think that caring for my brain-dead patient satisfied some small part of this need to feel human, whether on the part of my patient before she died and who perhaps lived in expectation of becoming a donor one day, or on the part of her relatives who gave their consent to the procedure after she was declared brain dead. Either way, I connected with her or with her family.
My experience in the operating room serves as a warning to those eager to cut corners and replace doctors with AI machines willy-nilly. You don’t satisfy people’s urge to feel human by making the bridge to the next world a totally inhuman one.
The lesson extends far beyond organ donation. AI promises to make healthcare quicker, more precise, and error-free. To the degree that it replaces doctors and nurses, it portends a massive shift in medicine that seems to come every 30 years, when people are so overwhelmed by the burden of their own technological creations, they need every iota of their strength to adjust. Once again, with the advent of AI, advancements promise to put healthcare on an entirely new footing and, once again, the inevitable backlash will be there. So it was in the 1960s, when new medical technologies and procedures, such as home dialysis machines and coronary artery bypass surgery, improved life, and yet, during this same period, the medical profession’s reputation plummeted, as patients complained that doctors had grown cold and impersonal. So it was in the 1990s, when the rise of managed care promised greater efficiency at less cost, and a ‘win-win’ strategy anchored in preventive medicine, led to a patient rebellion against being treated like cattle, including their inability to choose their own doctor, and rushed visits to practitioners ‘on the plan’.
Now, in the 2020s, the stage is set for outrage, yet again. AI promises to elevate healthcare; but, to the degree that it replaces doctors and nurses, it also threatens to depersonalise patients and to wash off their distinctive colours until everyone has the same drab tint. In the crucible that looms, patients are going to rebel. And it is in the arena of organ donation, where they face death by machine, that they are likely to baulk loudest, and first.

Stories and literature
Laboratories of the impossible
By testing the boundaries of reality, Spanish-language authors have created a sublime counterpart to experimental physics
Joshua Roebke

Roaming rocks
Metamorphic rocks are our emissaries from the deep, travelling to alien realms and revealing the restless nature of Earth
Marcia Bjornerud

Philosophy of mind
The stories of Daniel Dennett
Often metaphorical and allusive, the philosopher’s work will long be remembered for how it grappled with everyday thought

Dance and theatre
Why the old man dances
Religious ritual to appease the gods or free expression of human agency? For the ancient Romans, dance could be both
Karin Schlapbach

Exploding the Big Bang
It was thought that science could tell us about the origins of the Universe. Today that great endeavour is in serious doubt
Daniel Linford

Future of technology
A linkless internet
In creating anonymous summaries, AI flattens out all the fascinating architecture of thought that makes the internet hum
Collin Jennings
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Practice and challenges for organ donation after medical assistance in dying: A scoping review including the results of the first international roundtable in 2021
Johannes mulder, hans sonneveld, dirk van raemdonck, james downar, beatriz domínguez‐gil, andrew healey, bruno desschans, arne neyrinck, alicia pérez blanco, ingeborg van dusseldorp, gert olthuis.
- Author information
- Article notes
- Copyright and License information
Correspondence , Johannes Mulder, Family Physician/Palliative Care Physician, Anesthesiology/Intensive Care department, Isala Hospitals, Zwolle, Family Medicine Center Dalfsen, the Netherlands. Email: [email protected]
Corresponding author.
Revised 2022 Sep 1; Received 2022 Jun 19; Accepted 2022 Sep 7; Issue date 2022 Dec.
This is an open access article under the terms of the http://creativecommons.org/licenses/by-nc-nd/4.0/ License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non‐commercial and no modifications or adaptations are made.
The procedure combining medical assistance in dying (MAiD) with donations after circulatory determination of death (DCDD) is known as organ donation after euthanasia (ODE). The first international roundtable on ODE was held during the 2021 WONCA family medicine conference as part of a scoping review. It aimed to document practice and related issues to advise patients, professionals, and policymakers, aiding the development of responsible guidelines and helping to navigate the issues. This was achieved through literature searches and national and international stakeholder meetings. Up to 2021, ODE was performed 286 times in Canada, the Netherlands, Spain, and Belgium, including eight cases of ODE from home (ODEH). MAiD was provided 17,217 times (2020) in the eight countries where ODE is permitted. As of 2021, 837 patients (up to 14% of recipients of DCDD donors) had received organs from ODE. ODE raises some important ethical concerns involving patient autonomy, the link between the request for MAiD and the request to donate organs and the increased burden placed on seriously ill MAiD patients.
Keywords: clinical research/practice, donors and donation: donation after circulatory death (DCD), guidelines, organ procurement, organ transplantation in general, patient safety, primary care, solid organ transplantation
Short abstract
This comprehensive overview of organ donation after medical assistance in dying and its attendant issues reports an increasing frequency of this practice internationally.
Abbreviations
donation after circulatory determination of death
medical assistance in dying
organ donation after euthanasia
organ donation after euthanasia from home
organ procurement organization (including donation coordinators)
1. INTRODUCTION
Since the first reported organ donation after euthanasia (ODE) in 2005, increasing numbers of patients requesting medical assistance in dying (MAiD) are asking to donate their organs after their death. s1 The development of ODE from home (ODEH) in 2017 might further improve the patient experience by eliminating the previous requirement for patients to encounter the hospital environment while conscious. ODE is of increasing importance for donors, representing up to 14% of donations after circulatory determination of death (DCDD). s2–s4 However, ODE is a complex procedure involving many unique ethical and logistical considerations and multiple stakeholders, including the patient and family, end‐of‐life care providers, and organ procurement organizations (OPO). To navigate these complexities, protocols have been developed to ensure ethical and compassionate end‐of‐life care and a positive donation experience for all concerned. This review aims to provide insight into international ODE practice by reviewing literature and holding stakeholder meetings on the practice of ODE, so as to advise patients, professionals, and policymakers in the context of their jurisdiction, aiding the development of responsible national guidelines.
The research objectives were formulated from the review's aim as follows: first, to provide an outline of ODE practice and current MAiD and DCDD practice relevant to ODE, second, to identify issues affecting MAiD patients, providers, and other stakeholders in the context of ODE practice, and, finally, to formulate guidance for providers and other stakeholders on the issues identified.
The objectives were researched in accordance with Arksey and O′Malley's scoping review methodology and the PRISMA‐ScR directions (Data S1 : research protocol). s5–s7 The literature search, covered bibliographic databases (Embase, Ovid/Medline, Web of Science, and Google Scholar) and information identified through other sources (webpages, guidance documents, news journals, citations, policy documents, guidelines, and protocols). The databases were queried for English language papers published between 2017 and 2021 relating to the current practice of MAiD and DCDD where relevant to ODE and for papers in any language published between 1995 and 2022 relating to ODE by an experienced research information specialist (ID). Two reviewers (JM and HS) independently screened study titles and abstracts from initial search results and subsequently reviewed full texts. They included papers explicitly involving analysis or description of at least one of the review objectives. Literature concerning death definitions, death criteria and assessment methods, ethical justification of MAiD and DCDD, the dead donor rule, and the recipient care pathway was excluded.
To supplement the literature search, national stakeholder consultations involving the eight countries, where MAiD with intravenous substances is currently (2021) performed, were carried out, charting current practices of MAiD and DCDD relevant to ODE and of ODE itself. These were followed by the first international roundtable of ODE stakeholders (Roundtable participants: Data S2 ) during the 2021 WONCA international family medicine conference, during which stakeholders from the eight countries discussed practices and issues related to the research objectives.
The data obtained from the literature search and national and international stakeholder consultations were analyzed in accordance with the objectives and allocated to major subthemes identified from the literature (JM and HS). Data were presented in tables and a narrative synthesis of the results addressing the study objectives, progressing from basic subject information to an overarching discussion of issues on ODE. No institutional review board approval was required, as the project involved charting existing rules and practices. Some of the ODE incidence data quoted have been published in a letter to the editor to this journal in October 2021. 185
3.1. Evidence analysis
A total of 2616 records (papers, abstracts, editorials, nonpublished articles, websites, guidance, and protocols) were identified up to February 6, 2022. After screening and eligibility assessment, 499 records were included in the narrative synthesis of the review (Figure 1 ). Thematic analysis yielded 10 clustered themes within the three main topics, MAiD, DCDD, and ODE: legislation, terminology, procedural aspects (protocols, eligibility, and safeguards), procedures in practice, and, specifically for ODE, desirability and appropriate care aspects. The key issues identified were death pathway concerns (consent, end‐of‐life care, guidelines, and access), MAiD in the DCDD death pathway, public trust, and providers' distress.
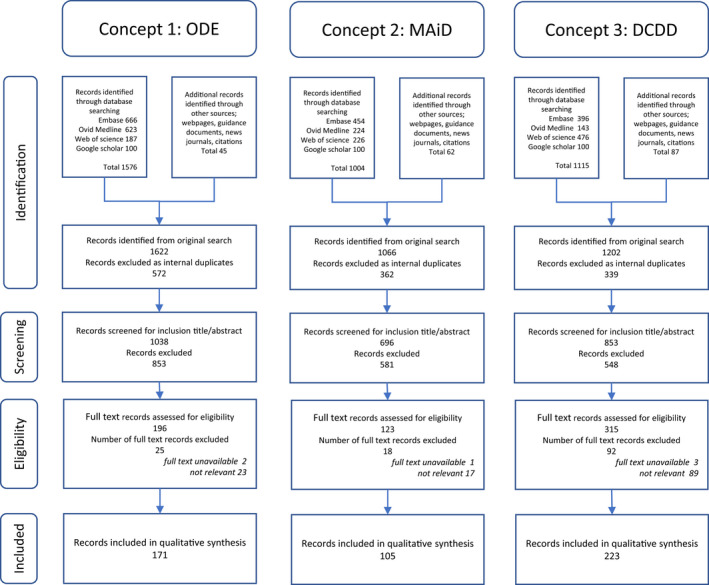
PRISMA‐ScR diagram. Three concepts are searched in four databases to satisfy the research aims: 1: all ODE literature between 1995 and 2022; 2: all review MAiD literature between 2017 and 2022; and 3: all review DCDD literature between 2017 and 2022. DCDD, donation after circulatory determination of death; MAiD, medical assistance in dying; ODE, organ donation after euthanasia
3.2. MAiD aspects relevant to ODE
3.2.1. legislation and terminology.
MAiD legislation has been discussed in many countries with varying levels of support and opposition. s8–s13 As of May 2022, 19 jurisdictions in eight countries have legislation permitting “MAiD by intravenous practitioner‐administration of lethal substances,” the form of MAiD compatible with ODE (Data S5 : Table B1). The main differences arise from the legals structure. Where MAiD is governed by the criminal code, the provider is only exempted from prosecution if MAiD is provided in accordance with appropriate care requirements. Where MAiD is decriminalized, postprocedural overview procedures are usually established. MAiD laws operate in conjunction with regional patient/professional medical treatment legislation to ensure appropriate end‐of‐life care alongside MAiD provision.
The legal act of practitioner administration of lethal MAiD substances with the intention of ending a patient's life at their voluntary, competent request was introduced in 2001 in the Netherlands, using the term euthanasia. s14,s15 Historical associations have caused reluctance to use this term universally, resulting in inconsistent use of terminology, with terms often encompassing both patient self‐administration and practitioner administration of MAiD substances. s16 For the purposes of this review, “MAiD” refers to practitioner administration of lethal substances.
3.2.2. Procedural aspects
The aim of the “MAiD‐patient care pathway” is a controlled, comfortable, and swift death, accomplished by circulatory arrest after induced acidosis, hypoxia, and cardiac depression, preceded by induced coma. s17,s18 MAiD legislation generally stipulates “reserved MAiD acts”, which may only be performed by the legal MAiD provider. These are administration of the MAiD substances and death declaration. The Netherlands, s17 Colombia, s19 and Spain s20 have specific national guidelines, whereas other jurisdictions have protocols. s21–s30 Provision commences with an intravenous coma‐inducing drug, followed by a paralytic agent and sometimes a cardioplegic agent. Typical coma inducers are thiopental and propofol. Nondepolarizing amino steroids are used as paralytic drugs and potassium chloride or bupivacaine as cardioplegic drugs.
The MAiD provider must declare death to bring the procedure to its legal conclusion. However, MAiD laws do not stipulate how death should be determined. Under general laws, death declaration is at the discretion of the attending physician. s31–s35 Where MAiD is governed by the criminal code, death is often required to be certified by a coroner.
The central eligibility criterion in all countries for MAiD is a required “symptom state” (Table 1 ). One of the main safeguards is that the MAiD provider must obtain and retain autonomous first‐person consent following a patient‐initiated request (Table 2 ). Autonomous means voluntary (competent and without coercion), well‐considered, and having unbiased information. Information should be provided about potentially burdensome premortal interventions such as intravenous cannula placement and MAiD assessments such as mandatory peer consultation by an unknown independent physician. Moreover, information provision must take account of the narrative aspects of autonomy, so information should be discussed in relation to the patient's expectations, values, and wishes. s36–s45 Ongoing end‐of‐life discussions, including ongoing assessment of the validity of the consent and care planning by the MAiD provider, are an important part of quality end‐of‐life care. s46–s56 Regulations generally allow for healthcare professionals having conscientious objections to performing MAiD.
MAiD as part of ODE: relevant eligibility criteria
Note : Eligibility criteria for MAiD that could potentially form part of ODE vary by jurisdiction.
Abbreviations: MAiD, medical assistance in dying; ODE, organ donation after euthanasia; RTS, information provided by roundtable stakeholders.
MAiD as part of ODE: relevant safeguards
Note : Required safeguards for appropriate/due care for MAiD that could potentially form part of ODE vary by jurisdiction.
The medical specialist should be specialized in the underlying condition.
In Quebec, only physicians are allowed to perform MAiD.
3.2.3. Practice
The 2020 incidence of MAiD, including self‐administration, was 17 217 (Data S5 : Table B2). Underlying medical conditions were predominantly oncological followed by neurological conditions. The practitioner administration mode of MAiD dominates. The time between MAiD induction and circulatory arrest averages 9 min, shortened by 1–2 min with the use of cardioplegia. s23,s27,s29,s30 Rare complications relate to venous access problems and the need for a second dose. s17,s18,s23,s27,s29,s30
Roundtable information
Stakeholders reported implementation of MAiD in Spain during this research, following its legalization in mid‐2021. No official figures for Spain are available yet (June 2022).
3.3. DCDD aspects relevant to ODE
3.3.1. legislation and terminology.
Organ donation legislation focuses on consent rules, prohibition of organ trade, donor care, premortal assessments, and the obligation to uphold the “dead donor rule,” originally devised in the USA, which states that patients must be deceased at the time of organ retrieval and the act of retrieval cannot be the cause of death. s42,s57–s59 DCDD, the donation scenario compatible with ODE, is not provided in Colombia or Luxembourg. s60–s62
Consent for the DCDD donor pathway must be obtained and retained by an OPO representative and may be given in advance, deemed (via opt‐out) or by a surrogate (in the absence of advance or deemed consent). In most jurisdictions, hospitals are required to notify the OPO of imminent deaths where organ donation may be possible.
DCDD is subdivided into uncontrolled (after unanticipated death) and controlled (after anticipated death) circumstances. s63 The latest DCDD classification refers to controlled DCDD (DCDD‐III) as “planned withdrawal of life sustaining therapy with expected cardiac arrest” with the footnote “This category mainly refers to the withdrawal of life‐sustaining therapy decision. Legislation in some countries allows euthanasia and subsequent organ donation described as the fifth category.” s63
Typical controlled DCDD involves unconscious, intensive care patients, with pathologies that have not caused death by neurological criteria, but where further treatment is no longer considered beneficial to the patient. s64 Withdrawal of life‐sustaining therapy is then performed with death as a foreseen but unintended consequence, allowing procurement of organs. s65,s66 Rare conscious DCDD involves patients who are dependent on life‐sustaining treatment but elect to cease treatment and donate their organs. s67–s70
In Colombia, cultural aspects prevent DCDD or, consequently, ODE from being introduced soon. In Luxembourg, discussions about introducing DCDD are ongoing.
3.3.2. Procedural aspects
Three “patient care pathways” are distinguished in DCDD, each with their own objectives, ethical justification, practice, stakeholder motivations, and consent procedures as follows: the death pathway leading to the donating patient's death, the donor pathway leading to the donation of the patient's organs, and the recipient pathway leading to organ implantation (Table 3 ). s71–s78 It is important to note that the death and donor pathways involve the same dying patient.
Patient care pathways and their primary objective to consider in different procedures
Note : Three “patient care pathways” are distinguished, each with their own ethical justification, consent procedures, and practice: the death pathway leading to the patient's death, the donor pathway leading to organ donation, and the recipient pathway leading to transplantation of donor organ(s). Several different pathways play a role within each of the procedures. To preserve autonomy in this ethically sensitive environment, they should intertwine as little as possible within the different procedures.
Abbreviations: DCDD, donation after circulatory determination of death; ICU, intensive care unit; MAiD, medical assistance in dying; N/A, not applicable; ODE, organ donation after euthanasia; WLST, withdrawal of life‐sustaining therapy.
When it is clear that treatment will not be medically effective and is not in accordance with the standard of care, the physician is not obliged to begin, continue, or maintain the treatment. To that extent, WLST is a medical decision. However, this decision should be inclusive, consultative, contemplative, and appropriately timed and preferably consensual with the family.
While practice varies, withdrawal of life‐sustaining therapy is generally initiated in the DCDD death pathway in intensive care or operating room by administration of sedatives/analgesics followed by withdrawal of vasoactive drugs and mechanical ventilation. s79–s82
The DCDD donor pathway involves premortal interventions for the recipient's benefit. Depending on which premortal interventions the country's legislation permits, these may include imaging, blood tests, invasive arterial blood pressure monitoring, heparin administration, and changing the setting where death takes place. s66,s79,s83–s88 Relatively new are postmortal regional perfusion procedures. s83,s89–s104
Death, currently defined as the moment of “permanent death,” must be awaited before organ procurement, to uphold the dead donor rule in the DCDD donor pathway. s42, s105–s108 With DCDD, the moment of permanent death is defined as the moment when circulation to the brain ceases following cardiac arrest and chances of spontaneous return of circulation are very small. s65 Circulatory arrest assessment methods and the wait time after arrest, required to establish “permanent death,” vary internationally (Table 4 ). Related OPO regulations and targets vary too. s109–s113
DCDD as part of ODE: relevant procedure aspects
Note : For DCDD, the required death assessment method and “wait time” after circulatory arrest before “permanent death” is established differ by country. The practice of deceased donation, DCDD donation, and specific DCDD organ procurement also varies significantly. In Colombia and Luxembourg, although MAiD is permitted, DCDD is not yet provided.
Abbreviations: DCDD, donation after circulatory determination of death; EC, echocardiogram; EKG, electrocardiogram; IABP, invasive continuous arterial blood pressure monitoring; MAiD, medical assistance in dying; ODE, organ donation after euthanasia.
Actual donors in 2020 per million population in a country.
Since 2021, the Australian and New Zealand Intensive Care Society (Anzics) Statement 2021 on Death and Organ Donation advises 5 min. 41
Eligibility for DCDD is mainly determined not only by consent, donor criteria, and logistics but also by jurisdiction‐specific circulatory death certification. The OPO representative must ascertain or obtain and retain consent for the DCDD donation pathway. s59 Where deemed or advance consent exists, this requirement is satisfied. s66,s114 In its absence, surrogate consent must be obtained from the family.
Premortal interventions and immediate postmortal procedures are justified for the recipient's benefit to optimize donor organ quality. s79,s83–s89,s115–s131 However, varying outcomes when evaluating premortal intervention benefit‐to‐harm ratios result in differences between provisions in different jurisdictions. s42,s66,s79,s84–s86,s114,s132,s133 Postmortal regional perfusion is under discussion in some jurisdictions, as it may pose death safeguard challenges or conflict with legal death definitions. s134–s145 For example, Australian legislation prohibits restoration of any circulation after death. s141 Healthcare professionals' conscientious objections to DCDD aspects are receiving increasing attention. s146–s149
3.3.3. Practice
DCDD incidence is increasing. s65,s150–s155 The period between starting the withdrawal of life‐sustaining therapy and permanent circulation arrest, which determines donor eligibility, varies from minutes to days (47% of DCDD cases meet the commonly applied 2 hours limit for donation eligibility) and is difficult to predict. s79–s81,s156–s161 Donation outcomes after DCDD are similar to outcomes following donation after neurological determination of death for kidneys, lungs, and pancreatic islets, but they are more variable for livers and hearts. s102,s140,s156,s162–s223
3.4. ODE/ODEH
3.4.1. legislation and terminology.
Current laws do not prohibit ODE, but do not mention the possibility either, and regulatory organs only make cautious statements, such as “Voluntary termination of life by means of euthanasia does not necessarily preclude organ and tissue donation.” 1 , 42 , 43 Or, for ODEH, “The committee regards the procedure in principle as a viable route, provided that it does not impede a careful establishment of death.” 44 This contrasts with the open support of “Right to Die” organizations, patient advocacy organizations, individual patients, and OPOs. 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55
The requirement for hospitals to notify OPOs of imminent deaths, in general, does not apply to MAiD patients, except in Ontario and Quebec in Canada where this is unclear. 56 , 57 , 58 ODE‐derived organs can only be allocated to jurisdictions where MAiD is permitted. 53
In 2017, the acronyms ODE and ODEH were coined to refer specifically to the total process that combines MAiD and DCDD, preserving the eligibility assessments and safeguards for both these subprocedures but integrating them in a way that places the emphasis on caring for the MAiD patient and the ethical perspectives and considerations this entails. 59 , 60 This emphasis is what distinguishes ODE from conscious (or unconscious) withdrawal of life‐sustaining therapy DCDD/DCDD‐III or DCDD‐V procedures. ODE should also be clearly distinguished from “living donation in anticipation of MAiD,” which has no regulatory or professional endorsement, and “MAiD by removal of organs in an anesthetized patient,” which constitutes homicide. 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72
All participants strongly supported the development of ODE and regarded ODE/ODEH as good universal terms for general adoption, as they are straightforward but indirect and have neutral semantics.
3.4.2. Procedural aspects
The major difference between ODE and typical DCDD‐III (or withdrawal of life‐sustaining therapy/DCDD) is the MAiD act in the DCDD death pathway, resulting in the foreseen and intended death of a conscious patient in the donor pathway, significantly affecting both pathways. ODEH involves additional deployment of a mobile team and mid‐procedure transport. 73 , 74
The Netherlands published a national guideline, based on a 2‐year Delphi‐like multistakeholder process organized by the Dutch Transplant Foundation (Table 5 ). Key considerations were peer‐reviewed, published, and presented to the Dutch house of representatives. 59 , 83 The guideline has two parts, consisting of a practice manual and background information, and replaces earlier manuals. It focuses on the MAiD patient in the death pathway during four phases: (1) decision‐making about the end of life, (2) preparations for the end of life, (3) end of life, and (4) organ donation and family grief counseling. The 2022 update includes the recommendation for the invasive blood pressure measurement and the ODEH option.
ODE guidelines and procedure summary
Note : Summary of the procedural aspects of ODE. Death declaration is more elaborate with DCDD, as the MAiD provider must receive advice on “permanent death” and sometimes more than one physician is required.
Abbreviations: EC, echocardiogram; EKG, electrocardiogram; DCD‐V, donation after circulatory death variant V; IABP, invasive continuous arterial blood pressure monitoring; MAiD, medical assistance in dying; ODE, organ donation after euthanasia; OPO, organ procurement organization; OTDT, organ and tissue donation and transplantation; Roc, rocuronium; RTS, information provided by roundtable stakeholders.
In Spain, a national protocol is expected in late 2022. In the meantime, a directive on “Summary OPO recommendations on donation after MAiD” was issued in July 2021 (RTS).
Canada published national policy guidance, based on a 2‐day forum organized by the Canadian Blood Services and operating together with existing protocols. 77 , 78 , 84 , 85 Key considerations were peer‐reviewed and published. The primary perspective is the DCDD donor patient pathway, with MAiD regarded as an end‐of‐life intervention with five steps: (1 + 2) MAiD request and determination of eligibility, (3) OPO referral and DCDD suitability assessment, (4) MAiD patient approach by OPO representative for consent and donor testing, and (5) hospital admission for premortal interventions and MAiD provision. Recent summits were held regarding the 2021 changes to MAiD legislation and the ODEH option.
Belgium published a national DCDD consensus by an expert group, organized by the Belgian Transplantation Society. 34 A chapter is dedicated to ODE, with the primary perspective of the donor pathway and focusing on the importance of preserving MAiD patient autonomy. More specific local protocols have been developed. 86
Spain commenced ODE practice following the legalization of MAiD but has no official publications yet. 79 , 80 , 81 , 82
In Spain, the National Transplant Committee established a working group in 2021 to develop an ODE/ODEH national protocol, which is currently subject to stakeholder consultation. In advance of this protocol, the National Transplant Organization released “recommendations for donor coordinator teams” for dealing with current requests. Key elements include MAiD pathway consent must precede donor pathway consent; MAiD plans take precedence over organ donation; patients can revoke donation consent at any time; organ donation may only be incorporated into the end‐of‐life process if consistent with the patient's wishes; and the patient's dignity and comfort must be ensured during the end‐of‐life process.
3.4.3. ODEH models
No ODEH guidelines have been published yet, but several models exist (Table 6 and Figure 2 ). With the Dutch model, an anesthesiologist/intensivist waits near the patient's preferred dying location. The procedure is initiated with midazolam sedation by the MAiD provider, with only the patient and their loved ones present for final farewells. After sedation, the anesthesiologist/intensivist is alerted and converts sedation to anesthesia with propofol, intubation, and the application of tidal ventilation. Subsequent continuous propofol administration is provided during transport and physiological conditions are maintained until MAiD death occurs in the hospital. Premortal interventions are performed in the hospital before MAiD administration. Death declaration by the MAiD provider according to permanent death criteria concludes the MAiD procedure and multiorgan donation follows. If mechanical, ventilated, or organ‐perfusion support unexpectedly fails during transport, the MAiD substances are administered immediately, initiating uncontrolled but comfortable death. Three variants have been performed. The first (Dutch‐I) variant differs from II and III by the absence of medic/paramedic attendance during farewells, the absence of vital monitoring when the patient is still conscious and subsequent transport with controlled anesthesia (variant III only).
Procedural aspects of ODEH models compared to WLST/DCDD and ODE
Note : Organizational aspects of ODEH compared with DCDD and ODE.
Abbreviations: DCD‐III, donation after circulatory death variant III; Fi, foreseen and intended death; Fn, foreseen but not intended death; M, multiple organ donation; MAiD, medical assistance in dying; N/A, not applicable; ODE, organ donation after euthanasia; ODEH, organ donation after euthanasia from home; RTS, information provided by roundtable stakeholders; S, single organ donation.
Sometimes vital monitoring is present during the initial sedation, while the patient is still conscious.
During transport the patient can be given sedation with sedatives or controlled anesthesia with propofol.
In Spain, sedation and analgesia prescribed by the attending physicians from the out‐of‐hospital transferring services are given according to standard practice. If the physician believes that the patient may become unstable after sedation, they will intubate and ventilate the patient under deep sedation during transportation to the hospital, where the patient will receive MAiD and DCDD.
Personal communication (Wilson Fareed Abdo, UMCN, Nijmegen, The Netherlands, June 6, 2022).
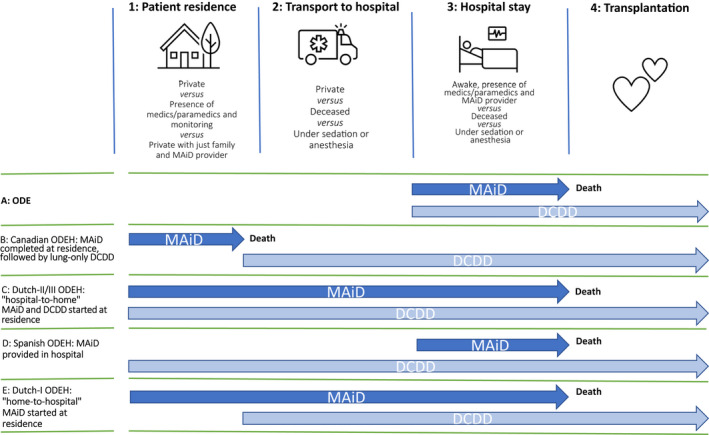
The ODE/ODEH modes. Steps 1–4 are the stages through which the patient progresses on the day when ODE/ODEH takes place: at home (1), transport to the hospital (2), hospital stay (3), and organ transplantation (4). The arrows mark the beginning and end of the MAiD and DCDD providers’ actions. For MAiD, this involves premedication, coma‐inducing medication, and finally, paralytics and, if applicable, cardioplegics. For DCDD, this involves attaching the monitor and observation, inserting lines, final assessments, and, once “permanent death” has occurred, commencing the transplantation procedure. The patient loses consciousness at stage 3 in procedure A and at stage 1 in other procedures. In B and E, the patient does not encounter the DCDD providers while conscious. Dutch‐II is identical to Dutch‐III except that transport is under sedation rather than anesthesia. DCDD, donation after circulatory determination of death; MAiD, medical assistance in dying; ODE, organ donation after euthanasia; ODEH, organ donation after euthanasia from home
With the Canadian model, a respiratory therapist and a physician wait close to the patient's preferred dying location. After MAiD substance administration at this location, the body is moved to the nearby ambulance, where EKG and apnea are monitored for 5 min. Death certification by the MAID provider and the second physician according to permanent death criteria concludes the MAiD procedure. After intubation and another 5‐min waiting period, a nonperfused, in situ lung preservation technique is applied and transport is initiated. At the hospital, lung retrieval is performed.
ODEH has already been performed in Spain, but not reported. The physician responsible for transport provides sedation and analgesia to the patient after final farewells at their home. A mobile intensive care unit then transfers the patient to the hospital, where the MAiD provider administers lethal medication. Following death certification by the MAiD provider, multiorgan retrieval takes place.
3.4.4. Eligibility and safeguards
Aside from logistical feasibility, eligibility for ODE/ODEH coincides with eligibility for MAiD and DCDD. The main safeguards involve the independent provision and maintenance of first‐person consent for both MAiD and DCDD. To safeguard autonomy, many jurisdictions prohibit or discourage OPO/physician‐initiated DCDD consent approaches or directed donation with ODE. 59 , 77 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98
Safeguarding end‐of‐life experience for MAiD patients in the ODE context requires active monitoring, given the potential for changes to the benefit/harm balance with these, sick, vulnerable patients, unlike unconscious withdrawal of life‐sustaining therapy patients. Examples include burdensome assessments during their final days of life, painful interventions, the presence of unknown healthcare teams during MAiD, a different setting for MAiD, changes to the family's experience, and bereavement due to the removal of the body for several hours. 59 , 77 , 92 , 95 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116
The Dutch guideline incorporates choices about varying levels of premortal intervention burden as an explicit part of the donor pathway consent process, with the patient deciding the level of burden they wish to endure and OPO representatives then deciding which organs can be procured based on the premortal interventions appropriate to this level of imposed burden. 59 In contrast to the regular approach of presenting premortal interventions and the promise of higher organ yield, this diminishes the risk of insufficiently well‐considered compromises to the end‐of‐life experience. 59 The Canadian guidance states “Based on their own comfort and preferences over how they wish to spend their last days, they may have questions and decline some investigations or donation interventions.” 77 With ODEH, some burdens and potential distress are reduced compared with ODE (Table 7 ).
Potential distress factors for persons involved within ODEH models compared to WLST/DCDD and ODE
Note : Sources of potential distress within the different procedures may originate from conscious and aware encounters by patients and healthcare providers. Awareness of PMIs is reduced when performed with unconscious patients.
Abbreviations: DCD‐III, donation after circulatory death variant III; DCDD, donation after the circulatory determination of death; N/A, not applicable; MAiD, medical assistance in dying; ODE, organ donation after euthanasia; ODEH, organ donation after euthanasia from home; OPO, organ procurement organizations; PMI, premortal intervention; RTS, information provided by roundtable stakeholders; WLST, withdrawal of life‐sustaining therapy.
In Spain, the MAiD provider is not involved in the transportation of the patient.
Reappraisal of the benefit/harm ratios of premortal interventions with ODE led to the removal of the requirement for potentially painful invasive blood pressure monitoring for death assessment in Canada, unlike the Netherlands, where the same discussions resulted in this requirement being re‐emphasized. 44 , 77 With ODEH, invasive blood pressure measurement is not burdensome as the patient is unconscious.
Concerns have been expressed regarding the use of sedatives during mid‐procedure ODEH transport as, unlike propofol anesthesia, these do not guarantee complete loss of awareness. 88 , 118
Healthcare providers' conscientious objections are a familiar topic within MAiD care pathways, but less common in DCDD death pathways, typically performed with the less controversial withdrawal of life‐sustaining therapy, and jurisdictions are developing protocols for this scenario. 59 , 77
The draft Spanish recommendations on ODE mention “prioritization of MAiD plans over organ donation” to prevent most end‐of‐life compromise. Stakeholders report that healthcare providers' conscientious objections are an active discussion topic.
3.4.5. Practice and donations
By 2021, ODE had been provided 286 times and ODEH 8 times, with the incidence rising annually (Table 8 ). The main underlying conditions were neurodegenerative and the most common MAiD provider was the general practitioner.
ODE/ODEH practice until 2021
Note : ODE/ODEH incidence, absolute and relative to deceased donation and DCDD. Due to COVID, no ODE took place in Belgium in 2021 (RTS). Contrary to popular belief, ODE is not provided in Luxembourg and Colombia (RTS). For relative incidence values, pre‐COVID data were used for better interpretation. Data provided by Eurotransplant statistics analysis for ODE, 2012–2021, Nederlandse transplantatie stichting (Sonneveld), the Netherlands; Eurotransplant statistics analysis for DCD‐V, 2005–2021, Transplant Centre University Hospitals Leuven (Desschans), Belgium; Statistical analysis for OTDT after MAiD, 2005–2021, Canadian Blood Service (LaHaie), Canada (excluding Québec), and Transplant Québec (Dupras‐Langlais), Québec, Canada; and Statistical analysis for ODE, 2021, Organización Nacional de Trasplantes (Pérez Blanco), Spain.
Abbreviations: DCDD, donation after the circulatory determination of death; DD, deceased donation; ODE, organ donation after euthanasia; ODEH, organ donation after euthanasia from home; RTS, information provided by roundtable stakeholders.
Spain started provision in 2020 and the first three occurrences of ODEH were in 2022 (data up to April 2022) (RTS).
The quality of ODE donor organs is at least comparable to organs retrieved following the withdrawal of life‐sustaining therapy/DCDD and even donation after neurological determination of death (Data S4 ).
This—perhaps not universally anticipated—similarity in organ quality may be due to earlier methodological bias in ischemia assessment, minimal lasting effects of MAiD substances, and underlying conditions and specific—pathophysiologic—effects related to donation after neurological determination of death. Experience with ODEH is limited, but there is no logical reason to assume inferiority.
Since 2005, 1136 organs have been donated, 988 of which were transplanted, to approximately 837 recipients resulting on average in 2.9 recipients receiving organs per ODE patient (Data S5 : Tables B3/B4 and Figure 3 ).
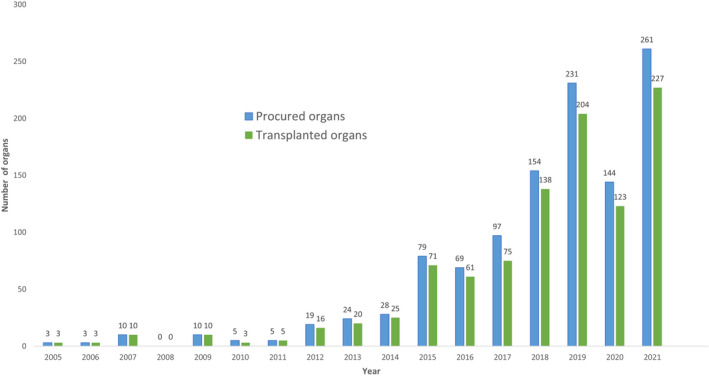
Procured and transplanted organs originating from ODE. Procured and transplanted organs originating from ODE annually. Data provided by: Eurotransplant statistics analysis for ODE, 2012–2021, Nederlandse transplantatie stichting (Sonneveld), the Netherlands; Eurotransplant statistics analysis for DCD‐V, 2005–2021, Transplant Centre University Hospitals Leuven (Desschans), Belgium; Statistical analysis for OTDT after MAiD, 2005–2021 Canadian Blood Service (LaHaie), Canada (excluding Québec) and Transplant Québec (Dupras‐Langlais), Québec, Canada; Statistical analysis for ODE, 2021, Organización Nacional de Trasplantes (Pérez Blanco), Spain. DCD‐V, donation after circulatory death variant V; MAiD, medical assistance in dying; ODE, organ donation after euthanasia; OTDT, organ and tissue donation and transplantation
3.4.6. Desirability and appropriate care
Eligibility criteria and safeguards should ensure the highest quality of care with medical procedures. The remaining challenges mentioned in the literature can be characterized as issues of desirability and appropriate care (also called due care). 136
The first ODE patient from Belgium told her GP in 2005: “I want to donate my organs. That way, I'll still be doing something good with this body of mine.” 119 A Dutch ODEH patient expressed the same sentiment in 2017: “I want to donate to be able to do something good with the diseased body that's also leading me to choose euthanasia.” 48 , 89 In 2019, a Canadian ODEH patient explained that “he wanted to save another person's life after seeing so many traumatic deaths on the job (being a policeman),” and an ODE patient “…kept pushing even though she wasn't able to speak (anymore).” 45 , 46 Many more patients have voiced this desire publicly. 45 , 46 , 47 , 52 , 137 These patients appear to have a dual perception of their bodies: instrumental as an object for donation and manifesting as a subject in the world. 138 , 139 , 140 This can be reformulated as “the severely diseased body that causes the loss of hope and suffering is, at the same time, a source of something good,” perhaps linked with the idea of a “body project,” indicating the importance of the body as a site for personal preferences and decisions. 48 , 59 , 119 , 139 , 141 , 142 Such a “body project” may contribute to the donor's moral identity, in which they see the body as a useful source for others in need, expressing something about their moral character. 139 , 143 For end‐of‐life care stakeholders, the desirability of ODE coincides with the last wishes of the MAiD patient. 46 , 144 , 145 Recipients and their stakeholders welcome the addition of donor organs. Public opinion is still evolving, but a Canadian survey has shown strong support for ODE. 77 The main concerns about appropriate care center around two issues: the potential for the MAiD decision and the organ donation decision to influence one another and premortal interventions and their effect on the MAiD patient's end‐of‐life experience in the donor pathway (Data S5 : Table B5).
All participants acknowledged that the practice of ODE has overwhelmingly been driven by MAiD patients wishing to donate.

4. DISCUSSION
The current practice of ODE, and of MAiD and DCDD where relevant to ODE, is outlined in Section 3 . The main issues and concerns identified are addressed here.
4.1. Donor pathway concerns
4.1.1. consent.
Resolving the consent issues in ODE starts with the recognition that the death pathway and donor pathway in DCDD involve the same potentially influenceable MAiD patient. There are several reasons why it is inherently impossible to adhere to the strict principle of separation of care pathways, as advocated in the withdrawal of life‐sustaining therapy/DCDD practice to minimize the risk of influence. 91 , 96 , 110 , 146 , 147 , 148 , 149 With the withdrawal of life‐sustaining therapy/DCDD, an unconscious patient's decision to donate is not subject to influence if deemed/advance consent is present. In its absence, approaching the family to obtain surrogate consent is explored. Besides, within the death pathway, the withdrawal of life‐sustaining therapy decisions with an unconscious patient is inherently nonpatient‐dependent. The only potential for influence on this decision lies in the interaction between the patient's intensive care team/surrogate decision‐maker and OPO representatives.
With ODE, there are multiple opportunities for considerations in one pathway to influence a decision made in the other. Current guidance indicates that the MAiD decision should precede discussion of organ donation and only after consent for MAiD is confirmed may the OPO representative approach the MAiD patient to obtain and retain consent for the donor pathway. But, from that moment on, the potentially influenceable patient (in contrast to the withdrawal of life‐sustaining therapy patient) not only decides on providing and maintaining donor pathway consent but also on maintaining the MAID death pathway consent. There is an inevitable intertwining of care pathways and ongoing interactions with the OPO representatives/donor coordinator might influence the donor to maintain consent for MAiD and donation. The MAiD provider needs to understand the dynamics of human decision‐making and the inherent psychological processes of altruism and social desirability that could uniquely influence the MAiD consent process in the context of ODE (but not when MAiD is the only consideration). 146 , 150 , 151 , 152 , 153 , 154
First‐person consent with ODE respects the interests of autonomy better than deemed or surrogate consent. Some ODEH modes enhance this, as patient care pathways do not intertwine on the final day while the patient is still conscious. 73 , 74 , 154
To anticipate the pathway interaction risks, the Dutch guideline advocates minimal contact between OPO representatives and the conscious MAiD patient. 59 Communication should be confined to providing donor pathway information, without the aim of conversion to ODE or retaining consent for DCDD, and MAiD should not be discussed at all. 111 With ODE, end‐of‐life care should remain the responsibility of the primary patient care team. From this perspective, labeling the donor consent process as “end‐of‐life care” could lead to coercion‐sensitive confusion. The Canadian guidance advocates specialized OPO representatives to deal with ODE practice. 77
Forum experts expressed concerns about unsolicited approaches to obtain donor pathway consent by treating physician or OPO and the idea of “directed donation” in ODE because of the potential to exacerbate the influence of donation pathway considerations on the MAiD decision. Some experts added that no information should be given on organ allocation, due to the risk that knowing how many people their organs could help will prevent the MAiD patient from feeling absolute freedom to change their mind right up until the last time they are asked whether they wish to proceed, just before substance administration.
4.1.2. End‐of‐life care
For the MAiD patient in the donor pathway, unlike the unconscious withdrawal of life‐sustaining therapy patient, every premortal intervention is potentially burdensome. The literature agrees that for valid donor pathway consent, all premortal interventions for the recipient's benefit must be discussed with the MAiD patient in terms of their necessity and impact on the end‐of‐life experience, bearing in mind the patient's aims and wishes for the end‐of‐life experience. Every premortal intervention should be carefully analyzed to establish its benefit/harm ratio. With some ODEH modes, the burden is diminished, as most premortal interventions can be performed while the patient is anesthetized.
Some experts advocated, “always ask the patient (which premortal interventions they want), they will tell you what they want,” but others added, “this only works when the patient is first provided with transparent and unbiased information (on premortal interventions).” Another issue considered was how far one should push the limits of DCDD practice to adapt to the addition of MAiD. It was felt that failing to adapt premortal interventions as much as possible would create an “all or nothing” model that would put patients under pressure and invariably fail to respect patient autonomy, either by forcing them to accept premortal interventions they do not want or by denying them the opportunity to donate. Ultimately, participants felt that the focus should be on the obligation to satisfy the patient's wishes when they are feasible and valid, and organ yield as a secondary benefit.
4.1.3. ODE guidelines
ODE is an exceptional procedure, presenting legal, ethical, and operational challenges and requiring dedicated guidance that will gain and retain society's confidence. If protocols are diffuse in their aims or burdened by earlier habits and ways of thinking, quality of care could be compromised and trust lost. 96 , 153 , 155 Professionals need to recognize each other's different roles and interests and coordination of this should be incorporated in a guidance document. Separate classifications of ODE‐related DCDD (DCDD‐V) and ODE‐related MAiD aid this objective. 156 , 157
“Do we only need guidelines to fill up the gaps between laws?” was asked. The conclusion was yes because the laws do not prohibit ODE but do not provide legal certainty in performance either; and no, as both MAiD and OPO providers need to make considerable changes to their usual practice in order to implement ODE.
4.1.4. Death
Upholding the dead donor rule and the need for viable organs led to the “permanent” death definition within DCDD. However, permanent does not equal irreversible, leaving a window for rare, undesired autoresuscitation. 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 Also, dying sometimes takes longer than expected. From the perspective of the MAiD‐patient care pathway, which focuses on swift and comfortable death, the occurrence of delayed death or autoresuscitation would suggest an insufficient first dose and a second dose should follow. In the withdrawal of life‐sustaining therapy patient care pathway, focused on ending mechanical, ventilated, or organ‐perfusion support when no longer considered beneficial to the patient while preventing discomfort, this occurrence signifies a prolonged dying phase and circulatory arrest (or its return) must be awaited. In the DCDD donor patient care pathway, focused on donor organ care without harming the donor, this occurrence also signifies a prolonged dying phase and death must be awaited (or awaited again) for the dead donor rule to be fulfilled before procurement can commence. Within ODE, the most feasible option in the event of delayed death seems to be for the MAiD provider to administer a second dose of MAiD substances after a predefined fixed period and for the OPO providers to wait for permanent death to occur (or reoccur).
Participants felt that diminishing undesired occurrence strengthens the case for use of cardioplegics, in the interests of both MAiD patient and recipient.
4.1.5. Access
The main limitation to ODE access is the limited number of jurisdictions (19) allowing practitioner‐administered MAiD. 79 , 166 , 167 , 168 , 169 , 170 The MAiD “stopcock infusion procedure,” available in some jurisdictions 42 and involving the patient self‐administering the intravenous MAiD substances, might be acceptable in more jurisdictions as it qualifies as assisted suicide and could be used with ODE.
DCDD access is also limited (17 countries). 171 DCDD incidence as a proportion of total deceased donations varies significantly by jurisdiction and further acceptance might increase access.
ODE incidence in jurisdictions permitting MAiD was 37 in 2020, while MAiD incidence was 16 977. With an estimated 10% eligibility, this would put utilization at roughly 2%, possibly due to a lack of familiarity and acceptance, the need for guideline development, and implementation. 172
Regional ODE access aspects include imminent‐death eligibility criteria for MAiD in New Zealand and Australia, the prereview requirement in Spain, and the legal default of self‐administration in some Australian jurisdictions.
The slow rise in ODEH access is probably related to the extra effort and logistics involved. Changes to standard performance take time to be accepted and embraced but, here again, patient demand probably becomes an important driver.
4.2. MAiD in the DCDD death pathway
One of the primary MAiD eligibility criteria is “unbearable suffering with no prospect of relief,” and the MAiD provider must be able to demonstrate this convincingly. A donation as a primary reason, that is, MAiD patients thinking that they are more valuable dead than alive, would be unacceptable and harm the MAiD cause. 112 , 146 , 150 , 151 , 152 , 173
4.3. Providers' distress and conscientious objections
Appropriate care involves safeguards to deal with professional distress and conscientious objections by healthcare providers. Within typical withdrawal of life‐sustaining therapy/DCDD, OPO representatives already describe approaching family members as the most stressful part of their job, which might be exacerbated by interactions with the conscious MAiD donor. 174 Concerns can extend to all involved. 175 , 176 , 177 Literature emphasizes the importance of healthcare providers participating voluntarily in the ethically sensitive environment of ODE. 111 , 174 , 176 , 178 , 179 , 180 , 181 , 182 , 183 , 184
Participants strongly supported voluntary involvement in the procedure.
4.4. Public trust and acceptance
Appropriate care with respect to public trust involves obtaining and maintaining society's support for a procedure like ODE. Society must be confident that the principle of nonmaleficence dominates medical practice and that the patient's needs always take priority over any organs they might donate after death. 126 , 155 If a public misperception arises that ODE is aimed at increasing organ procurement, this confidence will be rapidly lost. 71 , 127 , 128 , 129
To ensure that ODE remains ethically justifiable, the procedure should remain a MAiD patient care–driven process, with the maximum adaptation of donation processes and minimal intrusion and impairment for end‐of‐life care.
4.5. Limitations and strengths
As is inherent with a scoping review compared with a systematic review, the quality of the studies included was not assessed and the synthesis is descriptive. Our search algorithm included various terms previously used to describe ODE, but others may exist. While our review included any article in any language on ODE, our search was conducted using English terms. Presubmission of the protocol was to local authorities and not as a journal submission, as is becoming more routine.
The review had a broad and inclusive search strategy, with evidence screened by two investigators at both titles/abstract and full‐text levels. National stakeholder meetings and an international roundtable enabled consultation with stakeholders of all countries permitting practitioner‐administered MAiD to validate the search results. Together with an interactive manuscript review, this all contributed to the validity.
5. CONCLUSIONS
As of 2021, ODE had been provided 286 times (including ODEH) in Belgium, the Netherlands, Canada, and Spain, donating 1131 organs to 837 recipient patients. Incidence is rising and ODE now represents a significant proportion of DCDD donations. MAiD and donor stakeholders regard ODE as desirable but emphasize the need for guidance and safeguards for appropriate care. Challenges for providers are obtaining autonomous, independent first‐person consent to MAID and DCDD and retaining this until provision and incorporating the varying jurisdictional requirements.
This comprehensive review of ODE/ODEH and MAiD/DCDD practice in relation to ODE/ODEH, and the issues involved may assist patients, professionals, and policymakers and aid the development of responsible protocols and guidelines.
5.1. Research gaps and further actions
This review shows several topics requiring further research (Data S5 : Table B6).
AUTHOR CONTRIBUTIONS
JM and HS: Conceptualization and methodology design, literature search, data collection and data analysis, project administration, data interpretation and writing, and review and editing. DR, AN, BD, KW, AH, AP, and BD: Investigation, data collection and analysis, data interpretation and writing, and review and editing. JD, GO, and ID: data interpretation and writing and review and editing. All authors and roundtable participants read and approved the final manuscript.
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation .
The tables providing background information on ODE are preceded with the letter B and contained in Background Tables B1‐B6 .
The references up to paragraph 3.4 and for the background tables, which largely provide background information on ODE, are preceded with the letter s and listed in Data S3 .
Supporting information
Mulder J, Sonneveld H, Van Raemdonck D, et al. Practice and challenges for organ donation after medical assistance in dying: A scoping review including the results of the first international roundtable in 2021. Am J Transplant. 2022;22:2759‐2780. doi: 10.1111/ajt.17198
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created during this review.
- 1. Euthanasia Code 2018: review procedures in practice. Regional euthanasia review committees. Published online 2018. Accessed May 20, 2022. https://www.euthanasiecommissie.nl/binaries/euthanasiecommissie/documenten/brochures/brochures/euthanasiecode/2018/euthanasia‐code‐2018/EuthanasieCode_2018_ENGELS_def.pdf
- 2. Termination of life on request and assisted suicide act. WTL Published; 2001. Accessed May 20, 2022. https://wetten.overheid.nl/BWBR0012410/2020‐01‐01 [ Google Scholar ]
- 3. The Belgian Act on Euthanasia of May, 28th 2002. Ethical Perspect. 2002;9(2–3):188‐335. doi: 10.1163/157180903770847599 [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Project de loi relatif à l'euthanasie. Doc 501488/001. Chambre Des Représentants de Belgique. Published; 2002. Accessed May 20, 2022. www.worldrtd.org/BelgiumLawTransl.html [ Google Scholar ]
- 5. Law of March 16, 2009 on palliative care, advance directive and end‐of‐life support. Journal officiel du Grand‐Duché de Luxembourg. Accessed May 20, 2022. http://legilux.public.lu/eli/etat/leg/loi/2009/03/16/n1/
- 6. Bill C‐14, House of Commons of Canada. Accessed May 20, 2022. https://parl.ca/DocumentViewer/en/43‐2/bill/C‐14/first‐reading
- 7. Bill C‐7, House of Commons of Canada. Accessed May 20, 2022. https://www.parl.ca/DocumentViewer/en/43‐1/bill/C‐7/first‐reading?col=2
- 8. Pesut B, Thorne S, Wright D, et al. Navigating medical assistance in dying from bill C‐14 to bill C‐7: a qualitative study. BMC Health Serv Res. 2021;21:1195. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Simmons JG, Reynolds G, Kekewich M, Downar J, Isenberg SR, Kobewka D. Enduring physical or mental suffering of people requesting medical assistance in dying. J Pain Symptom Manage. 2022;63:244‐250.e2. doi: 10.1016/j.jpainsymman.2021.08.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Wiebe E, Green S, Wiebe K. Medical assistance in dying (MAiD) in Canada: practical aspects for healthcare teams. Ann Palliat Med. 2021;10(3):3586‐3593. doi: 10.21037/apm-19-631 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Pesut B, Wright D, Thorne S, et al. What's suffering got to do with it? A qualitative study of suffering in the context of medical assistance in dying (MAID). BMC Palliat Care. 2021;20:174. doi: 10.1186/s12904-021-00869-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Voluntary Assisted Dying Act 2017 61/2017 Victoria. Accessed May 20, 2022. https://www.legislation.vic.gov.au/in‐force/acts/voluntary‐assisted‐dying‐act‐2017/003
- 13. McLaren C, Jennens R, Kosmider S, et al. Voluntary assisted dying (VAD) in Victoria: a case series of patient characteristics 2019‐2021. Asia Pac J Clin Oncol. 2021;17(SUPPL 9):79‐80. doi: 10.1111/ajco.13716 32969171 [ DOI ] [ Google Scholar ]
- 14. Voluntary Assisted Dying Act 2019 Western Australia. Accessed May 20, 2022. https://www.legislation.wa.gov.au/legislation/statutes.nsf/law_a147242.html
- 15. End‐of‐Life Choices (Voluntary Assisted Dying) Act 2021 Tasmania 2021. Accessed May 20, 2022. https://www.legislation.tas.gov.au/view/html/asmade/act‐2021‐001
- 16. Voluntary Assisted Dying Bill 2021, Queensland. Published online 2021. Accessed May 20, 2022. https://www.health.qld.gov.au/system‐governance/legislation/voluntary‐assisted‐dying‐bill
- 17. A legal framework for voluntary assisted dying. Queensl law reform Comm . Accessed May 20, 2022. https://www.qlrc.qld.gov.au/__data/assets/pdf_file/0003/681132/qlrc‐report‐79‐report‐summary.pdf
- 18. South Australia Voluntary Assisted Dying Act 2021. Accessed May 20, 2022. https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/services/primary+and+specialised+services/voluntary+assisted+dying
- 19. Voluntary Assisted Dying Bill 2021, New South Wales. Accessed May 20, 2022. https://parliament.nsw.gov.au/bills/Pages/bill‐details.aspx?pk=3891
- 20. Bill No. 46–7 of 3/24/21 on the Organic Law to Regulate Euthanasia. Boletín Oficial del Estado.
- 21. End of Life Choice Act New Zealand 2019. 2019. Accessed May 20, 2022. https://www.health.govt.nz/our‐work/life‐stages/assisted‐dying‐service/end‐life‐choice‐act‐2019
- 22. Sentence c‐239/97, Ref. Expedient D‐1490, May 20, 1997, Republic of Colombia Constitutional Court. Accessed May 20, 2022. https://www.patientsrightscouncil.org/site/wp‐content/uploads/2015/05/Colombia_Court_Decision_05_20_1997.pdf
- 23. DMD Colombia Fundacion Pro Derecho a Morir Dignamente. Accessed May 20, 2022. https://dmd.org.co/ .
- 24. [Protocol for the application of the euthanasia procedure in Colombia], Ministerio de Salud y Protección Social, Bogotá, 2015. Accessed May 20, 2022. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/CA/Protocolo‐aplicacion‐procedimiento‐eutanasia‐ .
- 25. Resolution 1216 of 2015. Comités para hacer efectivo el derecho a morir con dignidad. Ministerio de Salud y Protección Social 20 April 2015. Accessed May 20, 2022. www.minsalud.gov.co/Normatividad_Nuevo/Resoluci%C3%B3n%201216%20de%202015.pdf . https://www.icbf.gov.co/cargues/avance/docs/resolucion_minsaludps_1216_2015.htm
- 26. Colombia high court SC‐233‐2021. Published online 1996.
- 27. O'Connor M, Philips J. Challenges of implementing voluntary assisted dying in Victoria. Australia Int J Palliat Nurs. 2020;26(8):425‐430. doi: 10.12968/ijpn.2020.26.8.425 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. White BP, Willmott L, Sellars M, Yates P. Prospective oversight and approval of assisted dying cases in Victoria, Australia: a qualitative study of doctors' perspectives. BMJ Support Palliat Care Published Online. 2021;bmjspcare‐2021‐002972. doi: 10.1136/bmjspcare-2021-002972 [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Hempton C. Voluntary assisted dying in the Australian state of Victoria: an overview of challenges for clinical implementation. Ann Palliat Med. 2021;10(3):3575‐3585. doi: 10.21037/apm-20-1157 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. International Registry in Organ Donation and Transplantation . 2021. Donation and transplantation institute. Accessed May 20, 2022. www.irodat.org
- 31. Holm AR, Courtwright A, Olland A, Zuckermann A, Raemdonck D. ISHLT position paper on thoracic organ transplantation in controlled donation after circulatory determination of death (cDCD). J Hear Lung Transplant. 2022;41(6):671‐677. doi: 10.1016/j.healun.2022.03.005 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Model protocol for post‐mortal organ and tissue donation. 2022 V5. Dutch Transplant Foundation (NTS). Accessed May 20, 2022. http://www.transplantatiestichting.nl/sites/default/files/modelprotocol_postmortale_orgaan‐_en_weefseldonatie.pdf
- 33. Lomero M, Gardiner D, Coll E, et al. European committee on organ transplantation of the Council of Europe (CD‐P‐TO). Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020;33(1):76‐88. doi: 10.1111/tri.13506 [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Evrard P. Donation after Circulatory Death (DCD) A Belgian Consensus. Les Éditions Namuroises; 2020. ISBN: 978–2–87551‐108‐9. [ Google Scholar ]
- 35. Shemie SD, Baker AJ, Knoll G, et al. National recommendations for donation after cardiocirculatory death in Canada: donation after cardiocirculatory death in Canada. CMAJ. 2006;175(8):S1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Kramer AH, Holliday K, Keenan S, et al. Donation after circulatory determination of death in western Canada: a multicentre study of donor characteristics and critical care practices. Can J Anesth. 2020;67(5):521‐531. doi: 10.1007/s12630-020-01594-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Organ Donation and Transplantation in Canada: Statistics, Trends and International Comparisons, Publication No. 2020‐28‐E 1 April 2020, Sonya Norris, Legal and Social Affairs Division Parliamentary Information and Research Service Published 2020. Accessed May 20, 2022. https://lop.parl.ca/site
- 38. Clinical Guidelines for Organ Transplantation from Deceased Donors, v1.6. The Transplantation Society of Australia and New Zealand. 2016. Accessed May 20, 2022. https://tsanz.com.au/guidelinesethics‐documents/organallocationguidelines.htm [ Google Scholar ]
- 39. Guideline Organ Donation after Circulatory Death; Patient Matters Manual for Public Health Organisations, New South Wales Government; 2020. Accessed May 20, 2022. https://www1.health.nsw.gov.au/pds/Pages/doc.aspx?dn=GL2020_007 [ Google Scholar ]
- 40. Matesanz Acedos R, Coll Torres E, Domínguez‐gil González B, Perojo Vega L. [DCDD in Spain: Current situation and recommendations] Donación en asistolia en España: Situación actual y recomendaciones. (Documento de Consenso Nacional 2012), Organización Nacional de Trasplantes Published online 2012. Accessed May 20, 2022. http://www.ont.es/infesp/Paginas/DocumentosdeConsenso.aspx
- 41. The Anzics Statement on Death and Organ Donation. Australian and New Zealand Intensive Care Society (ANZICS); 2021. Accessed May 20, 2022. https://www.anzics.com.au/death‐and‐organ‐donation/ [ Google Scholar ]
- 42. Guideline Provision Euthanasia and Assisted Suicide, Royal Dutch Medical Association (KNMG) and Royal Dutch Pharmacists Association (KNMP), 2021. Accessed May 20, 2022. https://www.knmg.nl/advies‐richtlijnen/dossiers/euthanasie.htm [ Google Scholar ]
- 43. Second Annual Report on Medical Assistance in Dying in Canada 2020, Health Canada, 2021, ISBN: 2563‐3643 . Accessed May 20, 2022. https://www.canada.ca/en/health‐canada/services/medical‐assistance‐dying.html
- 44. Determining death in organ donation after euthanasia. Gezondheidsraad, 2018. Accessed May 20, 2022. https://www.gezondheidsraad.nl/documenten/adviezen/2018/12/12/vaststellen‐van‐de‐dood‐bij‐orgaandonatie‐na‐euthanasie . 10.1007/s12445-017-1014-7 [ DOI ]
- 45. N.B. woman first in province to donate organs after MAID, 13/1/22, L. Brown, CTV News. Accessed May 20, 2022. https://atlantic.ctvnews.ca/n‐b‐woman‐first‐in‐province‐to‐donate‐organs‐after‐maid‐1.5737612
- 46. An Ontario man chose a medically assisted death at home. In a world first, he was able to donate his lungs. Megan Ogilvie, March 7 2021, Toronto Star. Accessed May 20, 2022. https://www.thestar.com/news/gta/2021/03/07/an‐ontario‐man‐chose‐a‐medically‐assisted‐death‐at‐home‐in‐a‐world‐first‐he‐was‐able‐to‐donate‐his‐lungs.html
- 47. Linkins LA. Shelly, MAiD and the purple parade. CMAJ. 2019;191(24):E668‐E669. doi: 10.1503/cmaj.190082 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. ALS patient donates his organs after euthanasia, EenVandaag [Documentary]; 2017, Reporter Gerie de Jong, Production Barbara van Gool, Johannes Mulder, Amsterdam, AVROTROS Productions. Published online 2017. Accessed May 20, 2022. https://www.npostart.nl/als‐patient‐donates‐his‐organs‐after‐euthanasia/11‐05‐2017/POMS_AT_8838678
- 49. The new Guideline organ donation after euthanasia provides more clarity for patients and professionals, 9/3/2017, Spierziekten Nederland. Accessed May 20, 2022. https://www.spierziekten.nl/nieuws/artikel/meer‐duidelijkheid‐orgaandonatie‐na‐euthanasie/terug/12/?tx_ttnews%5Byear%5D=2017&tx_ttnews%5Bpointer%5D=12
- 50. Enthoven L. Euthanasia and organ donation can be fullfilled together. Euthanasie en orgaandonatie kúnnen samengaan. Relevant. 2014;4:4‐6. Accessed May 20, 2022. https://www.nvve.nl/over‐ons/relevant [ Google Scholar ]
- 51. Schurink R, Organ Donation after Euthanasia, 2016. NVVE. Accessed May 20, 2022. https://www.nvve.nl/actueel/nieuws/week‐12‐orgaandonatie‐na‐euthanasie [ Google Scholar ]
- 52. Effting M. After euthanasia no donation in Radboud hospital. Volkskrant. 2015;6:feb. Accessed May 20, 2022. https://www.volkskrant.nl [ Google Scholar ]
- 53. Oosterlee A, Rahmel A. REC01.08 Ethics Committee (ETEC), Annual Report 2008. Eurotransplant International Foundation;2009. Accessed May 20, 2022. https://www.eurotransplant.org/annual‐reports‐archive/ [ Google Scholar ]
- 54. Halifax woman plans unforgettable gift for her husband to find after her death. Accessed May 20, 2022. https://www.cbc.ca/news/canada/nova‐scotia/shelly‐sarwal‐randy‐tressider‐meaghan‐smith‐song‐1.5254394
- 55. Dr. Shelly Sarwal, Her last project. Accessed May 20, 2022. https://herlastproject.ca/author/herlastproject/page/2/
- 56. A designated facility shall notify the Agency as soon as possible when a patient at the facility has died or a physician is of the opinion that the death of a patient at the facility is imminent by reason of injury or disease, Gift of Life Act, R.S.O. 199. Accessed May 20, 2022. https://www.ontario.ca/laws/statute/90h20
- 57. When informed of the imminent or recent death of a potential organ or tissue donor, the director of professional services of an institution operating a general and specialized hospital shall diligently (204.1), An Act Respecting Health Services and Social. Accessed May 20, 2022. http://legisquebec.gouv.qc.ca/en/document/cs/S‐4.2
- 58. Yazdani S, Buchman DZ, Wright L, Chandler JA. Organ donation and medical assistance in dying (MAID): ethical and legal issues facing Canada. Mcgill J Law Heal. 2017;11(2):59‐86. [ Google Scholar ]
- 59. Mulder H, Olthuis G, Siebelink M, Gerritsen R, van Heurn E. Multidisciplinary national guideline “Organ donation following euthanasia”; Practice manual and core document. Ned Tijdschr Geneeskd. 2017;161:D2135. Accessed May 20, 2022. https://www.ntvg.nl/artikelen/richtlijn‐orgaandonatie‐na‐euthanasie#popup‐abstract‐en w [ PubMed ] [ Google Scholar ]
- 60. Mulder J, Sonneveld JPC. Organ donation following euthanasia starting at home. Transpl Int. 2017;30(10):1075‐1076. doi: 10.1111/tri.13003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Bollen J, Shaw D, De Wert G, et al. Organ donation euthanasia (ODE): performing euthanasia through living organ donation. Transplantation. 2020;104(SUPPL 3):S298. doi: 10.1097/01.tp.0000700004.43157.0a [ DOI ] [ Google Scholar ]
- 62. Ely EW. Death by organ donation: euthanizing patients for their organs gains frightening traction. Intensive Care Med. 2019;45(9):1309‐1311. doi: 10.1007/s00134-019-05702-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Somerville M. Does it matter how we die? Ethical and legal issues raised by combining euthanasia and organ transplantation. Linacre Q. 2019;86(4):359‐365. doi: 10.1177/0024363919872623 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Truog RD, Miller FG, Halpern SD. The dead‐donor rule and the future of organ donation. N Engl J Med. 2013;369(14):1287‐1289. doi: 10.1056/NEJMp1307220 [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Lee GS, Potluri VS, Reese PP. The case against imminent death donation. Curr Opin Organ Transplant. 2017;22(2):184‐188. doi: 10.1097/MOT.0000000000000389 [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Morrissey PE. Organ donation and imminent death: pro position. Curr Opin Organ Transplant. 2017;22(2):179‐183. doi: 10.1097/MOT.0000000000000388 [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Cronin AJ. Death by donation: reflections on individual authorization, assisted suicide and organ donation. Transplantation. 2014;98(3):254‐256. doi: 10.1097/TP.0000000000000238 [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Shaw DM. Organ donation after assisted suicide: a potential solution to the organ scarcity problem. Transplantation. 2014;98(3):247‐251. doi: 10.1097/TP.0000000000000099 [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Bollen JAM, Shaw D, de Wert G, et al. Euthanasia through living organ donation: ethical, legal, and medical challenges. J Hear Lung Transplant. 2019;38(2):111‐113. doi: 10.1016/j.healun.2018.07.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Wilkinson D, Savulescu J. Should we allow organ donation euthanasia? Alternatives for maximizing the number and quality of organs for transplantation. Bioethics. 2012;26(1):32‐48. doi: 10.1111/j.1467-8519.2010.01811.x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Ely EW. Fake news on euthanasia and organ donation. Author's Reply Intensive Care Med. 2020;46(3):566‐567. doi: 10.1007/s00134-019-05845-1 [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Molchan K, Potter J. Further benefits of imminent death donation. Am J Transplant. 2021;21(9):3199‐3200. doi: 10.1111/ajt.16525 [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Mulder J, Sonneveld JPC. Organ donation after medical assistance in dying at home. CMAJ. 2018;190(44):E1305‐E1306. doi: 10.1503/cmaj.170517 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Healey A, Cypel M, Pyle H, et al. Lung donation after medical assistance in dying at home. Am J Transplant. 2021;21(1):415‐418. doi: 10.1111/ajt.16267 [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Mulder H. Organ donation after euthanasia and the general Practioner. Orgaandonatie na euthanasie en de huisarts, scientific conference proceedings Dutch College of General Practitioners (NHG). Huisarts Wet. 2018;7:1‐6. Accessed May 20, 2022. https://www.henw.org/artikelen/onderzoek‐nhg‐wetenschapsdag‐2018 [ Google Scholar ]
- 76. Mulder J, Organ donation after MAiD: the GP's perspective, LHV (Dutch National Association of General Practitioners) Conference, August 2018.
- 77. Downar J, Shemie SD, Gillrie C, et al. Deceased organ and tissue donation after medical assistance in dying and other conscious and competent donors: guidance for policy. CMAJ. 2019;191(22):E604‐E613. doi: 10.1503/cmaj.181648 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Canadian Medical Association . Correction: deceased organ and tissue donation after medical assistance in dying and other conscious and competent donors: guidance for policy. CMAJ. 2019;191(26):E745. doi: 10.1503/cmaj.190724 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Two euthanasia patients in Spain donated their organs, C. Kin, 22 November 2021, Euroweekly. Accessed May 20, 2022. https://euroweeklynews.com/2021/11/22/two‐euthanasia‐patients‐in‐spain‐donated‐their‐organs/
- 80. The National Transplant Organization of Spain, La Organización Nacional de Trasplantes (ONT). Accessed May 20, 2022. http://www.ont.es/home/Paginas/default.aspx
- 81. The transplant protocol after euthanasia, ready before April 2022. El protocolo de trasplantes tras la eutanasia, listo antes de abril de 2022. 29/11/21, T. Calahorra, Redaccion medica. Accessed May 20, 2022. https://www.redaccionmedica.com/secciones/sanidad‐hoy/eutanasia‐donacion‐organos‐7202
- 82. Spain has already transplanted the organs of 7 patients who requested euthanasia, España ya ha trasplantado los órganos de 7 pacientes que pidieron eutanasia, O Batres, 21/1/2022, Redaccion medica. Accessed May 20, 2022. https://www.redaccionmedica.com/secciones/sanidad‐hoy/espana‐ya‐ha‐trasplantado‐los‐organos‐de‐7‐pacientes‐que‐pidieron‐eutanasia‐9511
- 83. Letter to parliament on directive guideline organ donation after euthanasia, Minister Bruins informeert de Kamer over de Richtlijn Orgaandonatie na Euthanasie, 7/10/2019. Accessed May 20, 2022. https://www.tweedekamer.nl/downloads/document?id=42767863‐aa40‐4703‐af2e‐e1c8476a87d4&title=RichtlijnOrgaandonatienaEuthanasie.pdf
- 84. Donation after MAiD Toolkit for Healthcare Workers, BC Transplant. 2020. Accessed May 20, 2022. http://www.transplant.bc.ca/Documents/Statistics/MAiD‐Toolkit‐Final.pdf [ Google Scholar ]
- 85. Trillium Gift of Life Network . Organ and Tissue Donation Following Medical Assistance in Dying: Program Development Toolkit. Trillium Gift of Life Network; 2018. Accessed May 20, 2022. https://www.giftoflife.on.ca/en/professionals.htm [ Google Scholar ]
- 86. Donatie na euthanasie DCD cat.V, UZ Leuven, Donor werkgroep UZ Leuven, 1–10‐2017.
- 87. Detry O, Laureys S, Faymonville M‐E, et al. Organ donation after physician‐assisted death. Transpl Int. 2008;21(9):915. doi: 10.1111/j.1432-2277.2008.00701.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Sonneveld JPC, Mulder J. Organ donation after euthanasia starting a.t home is feasible. JAMA Surg. 2021;156(5):502. doi: 10.1001/jamasurg.2020.6674 [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Mulder JGH, Sonneveld JPC. Organ donation following physician‐hastened death at home. Ned Tijdschr Geneeskd. 2017;161:D1779. Accessed May 20, 2022. https://www.ntvg.nl/artikelen/orgaandonatie‐na‐euthanasie‐vanuit‐de‐thuissituatie [ PubMed ] [ Google Scholar ]
- 90. Tajaâte N, van Dijk N, Pragt E, et al. Organ donation after euthanasia starting at home in a patient with multiple system atrophy. BMC Med Ethics. 2021;22(1):120. doi: 10.1186/s12910-021-00686-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Van Bruchem‐Visser R, Van Dijk G, De Beaufort I. Organ donation after euthanasia, morally acceptable under strict procedural safeguards. Eur Geriatr Med. 2018;9:S325‐S367. doi: 10.1007/s41999-018-0097-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Allard J, Fortin MC. Organ donation after medical assistance in dying or cessation of life‐sustaining treatment requested by conscious patients: the Canadian context. J Med Ethics. 2017;43(9):601‐605. doi: 10.1136/medethics-2016-103460 [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Mulder H, Gerritsen R, Olthuis G, Abdo F. Unnecessary Turmoil (on Mandatory Approach of MAiD Patients) around New Donor Law and Donation after Euthanasia, Medisch Contact. 2020. Accessed May 20, 2022. https://www.medischcontact.nl/nieuws/laatste‐nieuws/artikel/onnodige‐onrust‐rond‐nieuwe‐donorwet‐en‐donatie‐na‐euthanasie.htm [ Google Scholar ]
- 94. Van Dijk G, van Bruchem‐Visser R, De Beaufort I, et al. Organ donation after euthanasia, morally acceptable under strict procedural safeguards. Clin Transplant. 2018;32(8):S325. doi: 10.1111/ctr.13294 [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Middleton C. Directed organ donation after medical assistance in dying: little to gain and much to lose. Can J Anesth. 2020;67(9):1310‐1311. doi: 10.1007/s12630-020-01679-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Middleton C. Organ donation after MAiD: it's not that simple. CMAJ. 2019;191(38):E1062. doi: 10.1503/cmaj.72983 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Casey GM, Kekewich M, Naik VN, Hartwick M, Healey A. A request for directed organ donation in medical assistance in dying (MAID). Can J Anaesth. 2020;67(7):806‐809. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Bollen J, ten Hoopen R, Ysebaert D, van Mook W, van Heurn E. Legal and ethical aspects of organ donation after euthanasia in Belgium and The Netherlands. J Med Ethics. 2016;42(8):486‐489. doi: 10.1136/medethics-2015-102898 [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Gruben V, Chandler J. Organ donation and medical assistance in dying in Canada. Transplantation. 2017;101(8):S14. doi: 10.1097/01.tp.0000524989.92926.3c [ DOI ] [ Google Scholar ]
- 100. Weiss MJ, van Beinum A, Harvey D, Chandler JA. Ethical considerations in the use of pre‐mortem interventions to support deceased organ donation: a scoping review. Transplant Rev. 2021;35(4):100635. doi: 10.1016/j.trre.2021.100635 [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Rasmussen SA, Barfield W, Honein MA. Voluntary euthanasia ‐ implications for organ donation. N Engl J Med. 2018;379(10):907‐909. doi: 10.1056/nejmp1809688 [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Chen E. Organ donation after assisted suicide: practically and ethically challenging. Transplantation. 2014;98(3):252‐253. doi: 10.1097/TP.0000000000000168 [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Dalle Ave AL, Shaw DM. Controlled donation after circulatory determination of death. J Intensive Care Med. 2017;32(3):179‐186. doi: 10.1177/0885066615625628 [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Rady MY, Verheijde JL, McGregor JL. Organ procurement and futile medical care. CMAJ. 2008;178(4):439‐440. doi: 10.1503/cmaj.1070171 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Murphy N, Weijer C, Smith M, et al. Controlled donation after circulatory determination of death: a scoping review of ethical issues, key concepts, and arguments. J Law Med Ethics. 2021;49(3):418‐440. doi: 10.1017/jme.2021.63 [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. O'Donovan K, Gilbar R. The loved ones: families, intimates and patient autonomy. Leg Stud. 2003;23(2):332‐358. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Ave ALD, Shaw DM. Author's Reponse to Kompanje letter to the editor. J Intensive Care Med. 2018;33(4):272‐273. doi: 10.1177/0885066617733663 [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Verheijde JL, Rady MY, McGregor JL. End‐of‐life care and organ procurement for transplantation: palliation or euthanasia? Crit Care Med. 2008;36(8):2481‐2482. doi: 10.1097/CCM.0b013e31817bfff2 [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Oczkowski SJW, Crawshaw DE, Austin P, et al. How can we improve the experiences of patients and families who request medical assistance in dying? A multi‐Centre qualitative study. BMC Palliat Care. 2021;20(1):185. doi: 10.1186/s12904-021-00882-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Shemie SD, Simpson C, Blackmer J, et al. Ethics guide recommendations for organ‐donation‐focused physicians: endorsed by the Canadian Medical Association. Transplantation. 2017;101(5):S41‐S47. doi: 10.1097/TP.0000000000001694 [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Ball IM, Sibbald R, Truog RD. Voluntary euthanasia ‐ implications for organ donation. N Engl J Med. 2018;379(10):909‐911. doi: 10.1056/NEJMp1804276 [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Gamondi C, Fusi‐Schmidhauser T, Oriani A, Payne S, Preston N. Family members' experiences of assisted dying: a systematic literature review with thematic synthesis. Palliat Med. 2019;33(8):1091‐1105. doi: 10.1177/0269216319857630 [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Citerio G, Cypel M, Dobb GJ, et al. Organ donation in adults: a critical care perspective. Intensive Care Med. 2016;42(3):305‐315. doi: 10.1007/s00134-015-4191-5 [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Ian M, Ball MD, Sibbald R, Robert D, Truog MD. Voluntary euthanasia — implications for organ donation. N Engl J Med. 2018;379(10):909‐911. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Goldberg R, Nissim R, An E, Hales S. Impact of medical assistance in dying (MAiD) on family caregivers. BMJ. 2021;11(1):107‐114. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Basmaji J, Weijer C, Skaro A, et al. Paving the road for the adoption of normothermic regional perfusion in Canada. Crit Care Explor. 2021;3(11):e0553. doi: 10.1097/cce.0000000000000553 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Verwiel J. Organ donaton after euthanasia. Orgaandonatie na euthanasie. Presentatie 22‐11‐2017, KNMG SCEN. Accessed May 20, 2022. https://www.knmg.nl/web/file?uuid=81b393f1‐4e0e‐4c18‐82f6‐42f77920fa0a&owner=5c945405‐d6ca‐4deb‐aa16‐7af2088aa173&contentid=69695
- 118. Myles P. Prevention of awareness during anaesthesia. Best Pr Res Clin Anaesthesiol. 2007;21(3):345‐355. PMID: 17900013. doi: 10.1016/j.bpa.2007.04.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Organ Donation after Euthanasia. Olivier Detry, Ignaas Devisch, Wim Distelmans, Liesbet Lauwereys, Lieve Thienpont, Dirk Van Raemdonck, Patrick Wyffels En Dirk Ysebaert, Belgische Transplantatie Vereniging, LevensEinde InformatieForum (LEIF).
- 120. Lossignol D, Lemaitre F, Hubert M. Euthanasia and organ donation, Euthanasie et don d'organes. Rev Med Brux. 2017;38(6):468‐469. [ PubMed ] [ Google Scholar ]
- 121. Ysebaert DK, De Greef K, Roeyen G, et al. Organ procurement after euthanasia procedure, respecting patient's will. Transpl Int. 2011;24:110‐130. doi: 10.1111/j.1432-2277.2011.01349.x [ DOI ] [ Google Scholar ]
- 122. Ysebaert D, Detry O, Verfaillie G, Mikhalski D, Van Raemdonck D. Organ donation after euthanasia on specific patients' request in Belgium. Transpl Int. 2015;28:114‐133. doi: 10.1111/tri.12700 [ DOI ] [ Google Scholar ]
- 123. Ysebaert D, Van Beeumen G, De Greef K, et al. Organ procurement after euthanasia: Belgian experience. Transplant Proc. 2009;41(2):585‐586. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Cras P, Van Beeumen G, Wijffels P, Heyvaert F, Ysebaert D. Non‐heart beating organ donor procedure after euthanasia in three neurological patients (abstract). Neurology. 2009;72(11):A122‐A123. [ Google Scholar ]
- 125. Detry O, Massion P, Ledoux D. Deceased organ donation in 2020. Rev Med Liege. 2020;75(5–6):276‐279. [ PubMed ] [ Google Scholar ]
- 126. Bernat JL. The boundaries of organ donation after circulatory death. N Engl J Med. 2008;359(7):669‐671. doi: 10.1056/NEJMp0804161 [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Spain joins the euthanize‐and‐organ‐harvest club, W. Smith, 30/1/22, National Review. Accessed May 20, 2022. https://www.nationalreview.com/corner/spain‐joins‐the‐euthanize‐and‐organ‐harvest‐club/ .
- 128. Schenderling J. They won't get my organs. Critical voices in the organ donation discussions. Tijdschr voor Gezondheidszorg en Ethiek. 2022;32(1):8. [ Google Scholar ]
- 129. Barras C. Medical euthanasia brings new questions for researchers. Nat Med. 2020;26(2):152‐154. doi: 10.1038/s41591-019-0696-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- 130. Rodríguez‐Prat A, Balaguer A, Crespo I, Monforte‐Royo C. The feeling of being a burden and the wish to hasten death among advanced patients: results of a meta‐ethnographic study. Palliat Med. 2018;32(1):203‐204. doi: 10.1177/0269216318769196 [ DOI ] [ Google Scholar ]
- 131. Goossens K, Van Bruchem‐Visser RL. Organ donation following euthanasia. Eur Geriatr Med. 2016;7:S109‐S110. [ Google Scholar ]
- 132. van Dijk G, Giezeman A, Ultee F, Hamers R. Organ donation after active euthanasia in a patient with a neurodegenerative disease. Ned Tijdschr Geneeskd. 2013;157(39):A6548. [ PubMed ] [ Google Scholar ]
- 133. van Wijngaarden AKS, van Westerloo DJ, Ringers J. Organ donation after euthanasia in The Netherlands: a case report. Transplant Proc. 2016;48(9):3061‐3063. doi: 10.1016/j.transproceed.2016.02.066 [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Erratum: Organ donation after medical assistance in dying ‐ Canada's first cases (New England Journal of Medicine). N Engl J Med. 2020;382(9):881. doi: 10.1056/NEJMx200006 [ DOI ] [ PubMed ] [ Google Scholar ]
- 135. Ball IM, Healey A, Keenan S, et al. Organ donation after medical assistance in dying ‐ Canada's first cases. N Engl J Med. 2020;382(6):576‐577. doi: 10.1056/NEJMc1915485 [ DOI ] [ PubMed ] [ Google Scholar ]
- 136. Ten Have H, Ter Meulen R, Van Leeuwen E. Textbook on Medical Ethics. Bohn Stafleu van Loghum; ISBN: 978–90–313‐9919‐2.; 2013. [ Google Scholar ]
- 137. van Steenbergen E. Arnold mag toch zijn nieren doneren, na zijn euthanasie. NRC ; 2015. [ Google Scholar ]
- 138. Slatman J, Flipse A. Bodyimages in Healthcare. In: Pieters T, Widdershoven G, eds. Basisboek Filosofie & Geschiedenis van de Gezondheidszorg. Boom; 2019:199‐218. [ Google Scholar ]
- 139. Shaw RM. Ethics, Moral Life and the Body. Sociological Perspectives. Palgrave Mac‐Millan; 2015. [ Google Scholar ]
- 140. Haddow G. The phenomenology of death, embodiment and organ transplantation. Sociol Health Illn. 2005;27(1):92‐113. [ DOI ] [ PubMed ] [ Google Scholar ]
- 141. Williams SJ. Modern medicine and the ‘uncertain body’: from corporeality to hyperreality. Soc Sci Med. 1997;45(7):1041‐1049. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Slatman J. Strange Body. About Medical Interventions and Personal Identity. Ambo/Anthos; 2008. [ Google Scholar ]
- 143. Schicktanz S. Why the way we consider the body matters ‐ reflections on four bioethical perspectives on the human body. Philos Ethics Humanit Med. 2007;2:30. doi: 10.1186/1747-5341-2-30 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Medical experts about the patient who changed their proffesion. This week GP Han Mulder. De Volkskrant. 30 December 2017. Accessed March 8, 2022. https://www.volkskrant.nl/mensen/peter‐wilde‐euthanasie‐en‐ook‐zijn‐organen‐doneren~b3589160/
- 145. Mendz GL, Kissane DW. Agency, autonomy and euthanasia. J Law Med Ethics. 2020;48(3):555‐564. doi: 10.1177/1073110520958881 [ DOI ] [ PubMed ] [ Google Scholar ]
- 146. Buturovic Z. Procedural safeguards cannot disentangle MAiD from organ donation decisions. J Med Ethics. 2021;47(10):706‐708. doi: 10.1136/medethics-2020-106456 [ DOI ] [ PubMed ] [ Google Scholar ]
- 147. Kalkman S, van Delden JJ. It takes two teams to save integrity. Ann Thorac Surg. 2016;102(6):1789. doi: 10.1016/j.athoracsur.2016.09.090 [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Corser N.. Re 17/1/2020 Organ donation after MAiD: what about presumed consent?
- 149. Matas AJ, Payne WD. Organ donation after euthanasia. JAMA Surg. 2020;155(4):924‐925. doi: 10.1002/jhbp.40 [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Buturovic Z. Embracing slippery slope on physician‐assisted suicide and euthanasia could have significant unintended consequences. J Med Ethics. 2020;47:257‐258. doi: 10.1136/medethics-2020-106089 [ DOI ] [ PubMed ] [ Google Scholar ]
- 151. Buturovic Z. Euthanasia and organ donation still firmly connected: reply to Bollen et al. J Med Ethics. Published Online First: 07 May 2021. doi: 10.1136/medethics-2021-107498 [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Bollen J, Vissers K, van Mook W. Dividing line between organ donation and euthanasia in a combined procedure. J Med Ethics. 2021;48(3):196‐197. doi: 10.1136/medethics-2021-107230 [ DOI ] [ PubMed ] [ Google Scholar ]
- 153. Baines L, Jindal RM. Organ donation after euthanasia: a dutch practical manual. Am J Transplant. 2017;17(3):842. doi: 10.1111/ajt.14044 [ DOI ] [ PubMed ] [ Google Scholar ]
- 154. Philpot SJ. Organ donation after circulatory death following voluntary assisted dying: practical and ethical considerations for Victoria. Crit Care Resusc. 2018;20(4):254‐257. [ PubMed ] [ Google Scholar ]
- 155. Abdo W. Organ donation after euthanasia. Handle with great care. Ned Tijdschr Geneeskd. 2014;158:A8617. [ PubMed ] [ Google Scholar ]
- 156. Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29(7):749‐759. doi: 10.1111/tri.12776 [ DOI ] [ PubMed ] [ Google Scholar ]
- 157. Evrard P. Belgian modified classification of Maastricht for donors after circulatory death. Transplant Proc. 2014;46(9):3138‐3142. doi: 10.1016/j.transproceed.2014.09.169 [ DOI ] [ PubMed ] [ Google Scholar ]
- 158. Hornby L, Dhanani S, Shemie SD. Update of a systematic review of autoresuscitation after cardiac arrest. Crit Care Med. 2018;46(3):e268‐e272. doi: 10.1097/CCM.0000000000002920 [ DOI ] [ PubMed ] [ Google Scholar ]
- 159. Hornby K, Hornby L, Shemie SD. A systematic review of autoresuscitation after cardiac arrest. Crit Care Med. 2010;38(5):1246‐1253. doi: 10.1097/CCM.0b013e3181d8caaa [ DOI ] [ PubMed ] [ Google Scholar ]
- 160. Dhanani S, Hornby L, van Beinum A, et al. Resumption of cardiac activity after withdrawal of life‐sustaining measures. N Engl J Med. 2021;384(4):345‐352. doi: 10.1056/nejmoa2022713 [ DOI ] [ PubMed ] [ Google Scholar ]
- 161. Sheth KN, Nutter T, Stein DM, Scalea TM, Bernat JL. Autoresuscitation after asystole in patients being considered for organ donation. Crit Care Med. 2012;40(1):158‐161. doi: 10.1097/CCM.0b013e31822f0b2a [ DOI ] [ PubMed ] [ Google Scholar ]
- 162. Koo CW, Helmick RA, Van Frank K, Gilley K, Eason JD. Incidence of autoresuscitation in donation after cardiac death donors. Am J Transplant. 2019;19:478‐479. doi: 10.1111/ajt.15405 [ DOI ] [ Google Scholar ]
- 163. Rady MY, Verheijde JL, McGregor J. “Non‐heart‐beating,” or “cardiac death,” organ donation: why we should care. J Hosp Med. 2007;2(5):324‐334. doi: 10.1002/jhm.204 [ DOI ] [ PubMed ] [ Google Scholar ]
- 164. Bernat JL, Bleck TP, Blosser SA, et al. Circulatory death determination in uncontrolled organ donors: a panel viewpoint. Ann Emerg Med. 2014;63(4):384‐390. doi: 10.1016/j.annemergmed.2013.05.018 [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Dhanani S, Hornby L, Ward R, et al. Vital signs after cardiac arrest following withdrawal of life‐sustaining therapy: a multicenter prospective observational study. Crit Care Med. 2014;42(11):2358‐2369. doi: 10.1097/CCM.0000000000000417 [ DOI ] [ PubMed ] [ Google Scholar ]
- 166. New South Wales draft non‐government bill to be introduced by: Mr A H Greenwich, MP Voluntary Assisted Dying Bill 2021 Contents. Accessed May 20, 2022. https://d3n8a8pro7vhmx.cloudfront.net/alexgreenwich/pages/10344/attachments/original/1632369383/Voluntary_Assisted_Dying_Bill_2021_%28pre‐introduction_draft%29.pdf?1632369383
- 167. Cormack L. Voluntary assisted dying bill passes final vote in NSW lower house, The Sydney Morning Herald. Accessed May 20, 2022. https://www.smh.com.au/politics/nsw/parliament‐at‐its‐best‐voluntary‐assisted‐dying‐bill‐passes‐final‐vote‐in‐nsw‐lower‐house‐20211126‐p59chi.html
- 168. Andreani T. Along the Italian route of end‐of‐life: the latest judicial evolution on assisted suicide. Bioethica. 2021;7(2):40. doi: 10.12681/bioeth.28158 [ DOI ] [ Google Scholar ]
- 169. Mroz S, Dierickx S, Deliens L, Cohen J, Chambaere K. Assisted dying around the world: a status quaestionis. Ann Palliat Med. 2021;10(3):3540‐3553. doi: 10.21037/apm-20-637 [ DOI ] [ PubMed ] [ Google Scholar ]
- 170. Dittborn M, Micolich C. Legalization of medically assisted death: its potential impact on the development of palliative care. Rev Med Chil. 2020;148(5):665‐673. [ DOI ] [ PubMed ] [ Google Scholar ]
- 171. Sixteenth rapport for the parlement: 2019 and 2020, Commission Nationale de Contrôle et d’ Evaluation de l’ application de la loi du 16 mars 2009 sur l’ euthanasie et l’ assistance au suicide. Published online 2020. Accessed May 20, 2022. https://sante.public.lu/fr/publications
- 172. Bollen J, Van Smaalen T, Hoopen RT, Van Heurn E, Ysebaert D, Van Mook W. Potential number of organ donors after euthanasia in Belgium. Jama. 2017;317(14):1476‐1477. doi: 10.1001/jama.2017.0729 [ DOI ] [ PubMed ] [ Google Scholar ]
- 173. Rodriguez‐Prat A, Balaguer A, Crespo I, Monforte‐Royo C. Feeling like a burden to others and the wish to hasten death in patients with advanced illness: a systematic review. Bioethics. 2019;33(4):411‐420. doi: 10.1111/bioe.12562 [ DOI ] [ PubMed ] [ Google Scholar ]
- 174. Silva E, Silva V, Hornby L, Almost J, et al. Burnout and compassion fatigue among organ and tissue donation coordinators: a scoping review. BMJ Open. 2020;10(12):e040783. doi: 10.1136/bmjopen-2020-040783 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 175. Buijsen MAJM. Discuss the relationship between physicians and pharmacists in the context of euthanasia. Ned Tijdschr Geneeskd. 2018;162:D2049. [ PubMed ] [ Google Scholar ]
- 176. Elmore J, Wright DK, Paradis M. Nurses' moral experiences of assisted death: a meta‐synthesis of qualitative research. Nurs Ethics. 2018;25(8):955‐972. doi: 10.1177/0969733016679468 [ DOI ] [ PubMed ] [ Google Scholar ]
- 177. Woods P, Schindel TJ, King MA, Mey A. Pharmacy practice in the domain of assisted dying: a mapping review of the literature. Res Soc Adm Pharm. 2020;16(3):267‐276. doi: 10.1016/j.sapharm.2019.05.012 [ DOI ] [ PubMed ] [ Google Scholar ]
- 178. Neades B. Healthcare professionals' experiences in applying presumed consent legislation in organ donation in three European countries: a phenomenological study. 2007. Accessed May 20, 2022. https://www.napier.ac.uk/research‐and‐innovation/research‐search/outputs/healthcare‐professionals‐experiences‐in‐applying‐presumed‐consent‐legislation‐in‐organ
- 179. Danet Danet A, Jimenez Cardoso PM, Pérez Villares JM. Emotional paths of professional experiences in transplant coordinators. Nefrologia. 2020;40:75‐90. [ DOI ] [ PubMed ] [ Google Scholar ]
- 180. Shaw D, Gardiner D, Lewis P, et al. Conscientious objection to deceased organ donation by healthcare professionals. J Intensive Care Soc. 2018;19(1):43‐47. doi: 10.1177/1751143717731230 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 181. Lamb C, Evans M, Babenko‐Mould Y, Wong C, Kirkwood K. Nurses' use of conscientious objection and the implications for conscience. J Adv Nurs. 2019;75(3):594‐602. doi: 10.1111/jan.13869 [ DOI ] [ PubMed ] [ Google Scholar ]
- 182. Ward V, Freeman S, Callander T, Xiong B. Professional experiences of formal healthcare providers in the provision of medical assistance in dying (MAiD): a scoping review. Palliat Support Care. 2021;1‐15. doi: 10.1017/S1478951521000146 [ DOI ] [ PubMed ] [ Google Scholar ]
- 183. Fujioka JK, Mirza RM, McDonald PL, Klinger CA. Implementation of medical assistance in dying: a scoping review of health care Providers' perspectives. J Pain Symptom Manage. 2018;55(6):1564‐1576.e9. doi: 10.1016/j.jpainsymman.2018.02.011 [ DOI ] [ PubMed ] [ Google Scholar ]
- 184. Variath C, Peter E, Cranley L, Godkin D, Just D. Relational influences on experiences with assisted dying: a scoping review. Nurs Ethics. 2020;27(7):1501‐1516. doi: 10.1177/0969733020921493 [ DOI ] [ PubMed ] [ Google Scholar ]
- 185. Mulder J, Sonneveld H, Healey A, Van Raemdonck D. The first international roundtable on “organ donation after circulatory death by medical assistance in dying” demonstrates increasing incidence of successful patient‐driven procedure. Am J Transplant. 2021;22(3):999‐1000. doi: 10.1111/ajt.16879 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (1021.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Home — Essay Samples — Economics — Donation — The Gift of Life: The Ethics of Organ Donation
The Gift of Life: The Ethics of Organ Donation
- Categories: Donation
About this sample

Words: 551 |
Published: Jun 13, 2024
Words: 551 | Page: 1 | 3 min read
Table of contents
The gift of life: organ donation, the critical need for organ donation, the life-saving benefits of organ donation, dispelling myths and misconceptions, ethical considerations and legislative support.
- United Network for Organ Sharing (UNOS). (2021). Facts About Organ Donation.
- World Health Organization (WHO). (2020). Global Observatory on Donation and Transplantation.
- Satel S., & Shwartz M.J.. (2019). When Altruism Isn’t Enough: The Case For Compensating Kidney Donors.
- Taylor J.S., & Caplan A.L.. (2018). The Ethics of Organ Transplants: A Philosophical Perspective.
- Lundin S., & Mouritsen R.. (2017). Organ Transplantation Policy: Ethical Perspectives on Systems Worldwide.

Cite this Essay
To export a reference to this article please select a referencing style below:
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr. Karlyna PhD
Verified writer
- Expert in: Economics
+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
1 pages / 618 words
2 pages / 790 words
1 pages / 448 words
4 pages / 1759 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Donation
Credit control is absignificant tool used by Reserve Bank of India. It is an important weapon of the monetary policy used to control demand and supply of money which is also termed as liquidity in the economy. Administers of the [...]
A few investigations have been directed on Dividend Policy by various researchers in various time frames. The connection of value instability and dividend yield is very noteworthy as contrast with different factors. (Asghar, [...]
A swap is an agreement between two counterparties to exchange two streams of cash flows the parties the cash flow streams Purpose of swap is to change the character of an asset or liability without liquidation Issuer of swap can [...]
After years of restricting foreign direct investment (FDI), governments in developing countries are now focusing on attracting external investors, spending large sums of money to entice foreign companies to their countries. In [...]
Lewis Lapham’s Money and Class in America describes how Americans use and value their money. He describes that money symbolizes a person’s success in life. He also puts in many religious aspects of money. However, not all people [...]
The financial crisis (2008): It is known as the worst economic tragedy since the Great Depression (1929).U.S. Investment bank and Lehman Brothers collapsed due to that crisis. The crisis was the outcome of many sequence events, [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
- Open access
- Published: 19 December 2024
The lived experience of living liver donors: A meta-synthesis of qualitative studies
- Li Dong 1 ,
- Zhiru Li 1 , 2 ,
- Feicui Lv 3 ,
- Li Zheng 1 &
- Fangyan Lu 1 , 3
BMC Nursing volume 23 , Article number: 925 ( 2024 ) Cite this article
48 Accesses
Metrics details
Living liver donors are frequently used for liver transplantation, but they experience many physical changes and psychological challenges. A better understanding of the experiences of liver donors can provide them with comprehensive and individualized patient-centred support and health care. Thus, this study sought to identify and synthesize existing qualitative studies to develop an in-depth understanding of the lived experiences of living liver donors.
A meta-synthesis was conducted and reported in accordance with the Enhancing Transparency in Reporting the Synthesis of Qualitative Research statement. Seven databases were systematically searched from inception to March, 2024, using a combination of Medical Subject Headings with keywords. The results were integrated using the three-step thematic synthesis method suggested by Thomas and Harden.
Nine studies met the inclusion criteria and were included in this meta-synthesis. Four themes emerged from the meta-synthesis: (1) facing a life-changing situation, (2) experiencing changes in interpersonal relationships, (3) coping with changes, and (4) achieving personal growth.
Conclusions
Living liver donors experienced physical, psychological, and social changes after surgery, striving to manage these challenges, highlighting the importance of coping strategies and ultimately achieving personal growth. Future nursing research should focus on psychological interventions, supportive measures, and the positive aspects of living liver donors to help them achieve the optimal quality of life.
Trial registration
PROSPERO CRD42022328947.
Peer Review reports
Introduction
Liver transplantation is the only effective intervention for end-stage liver disease. Despite the significant innovations in graft optimization and organ allocation processes over the past two decades, the shortage of available liver donor organs remains pressing [ 1 ]. This has led to a longer median time on the waiting list for transplantation. Data from the United Network for Organ Sharing (UNOS) shows that nearly 1,200 patients die every year while waiting for a liver transplant [ 1 ]. In response to the shortage of liver grafts from deceased donors, living donor liver transplantation (LDLT) has developed as an important alternative [ 2 ]. Influenced by cultural, socio-economic and other constraints, deceased-donor liver transplantation is predominant in Europe and America unlike Asia where LDLT accounts for the majority of liver transplants [ 3 ]. LDLT has the potential to increase the donor pool and decrease the waiting list mortality. What’s more, LDLT provides high-quality grafts for the recipients and potentially avoids dysregulation, or death due to changes in clinical status. Also LDLT has the advantage of scheduling transplantation as an elective procedure and selecting the best anatomical match [ 4 ].
In spite of LDLT’s advantages in long-term survival outcomes for recipients [ 4 ], it, as an invasive surgery, may cause donors to experience a series of complications after surgery, such as intra-abdominal haemorrhage, abdominal incision infection, bile leak, and even death in severe cases [ 5 ]. The donor complication rates reported vary significantly from 24 to 67% in the literature [ 6 ]. These postoperative complications not only affect the rehabilitation of a patient’s physical function but also challenge the recovery of the patient’s psychological functions to some extent, which decreases their long-term health-related quality of life [ 7 ]. Several studies [ 8 , 9 , 10 ] have reported poor postoperative psychological conditions in living liver donors, such as anxiety, depression, remorse, and post-traumatic stress disorder. The emotional stress associated with a donation may increase psychological vulnerability and lead to mental health problems. In addition, donation-related interpersonal relationships and financial strains were noted [ 11 , 12 ]. A study [ 13 ] conducted by Ordin et al. indicated that living liver donors need physical care and psychosocial support after surgery to cope with psychological, social, and financial problems. Thus, fully understanding and exploring the real lived experience of living liver donors can promptly identify the possible needs, which is conducive to providing targeted support interventions to help donors recover better and return to normal life.
The majority of previous studies regarding living liver donors after surgery have focused on immediate and long-term medical outcomes with quantitative methods, including investigations of surgical complications, psychological and socioeconomic complications, fast recovery strategies and health-related quality of life [ 14 ]. Due to the limitations of quantitative review studies in terms of understanding living liver donors’ everyday experiences, an increasing number of studies have explored the experiences of living liver donors through qualitative methods. These studies have employed various qualitative methodological approaches and have various foci (e.g., complications, the donor-recipient relationship, postoperative coping experiences, transplant information needs, and donation decision-making). However, the diversity of these qualitative studies and the differences in their findings have made it difficult to gain a fuller understanding of the real lived experiences of living liver donors. Therefore, this meta-synthesis aimed to analyse, interpret, and synthesize previous qualitative studies that explored living liver donors’ experiences.
Study design
We registered this qualitative meta-synthesis on PROSPERO (PROSPERO CRD42022328947) and reported our findings in accordance with the Enhancing Transparency in Reporting the Synthesis of Qualitative Research statement to strengthen the completeness of reporting [ 15 ]. We chose qualitative meta-synthesis because this research approach can preserve the meaning of individual studies while producing a new and integrated interpretation of findings and can help develop a theoretical and conceptual understanding and enhance the development of clinical practice and intervention design [ 15 ].
Search strategy
We conducted a comprehensive search of PubMed, Embase, Web of Science, Scopus, CINAHL, Cochrane, and PsycINFO databases for publications using a combination of Medical Subject Headings (MeSH) with keywords. After our first search, we conducted a manual search based on the citations of the included studies, relevant published systematic reviews, and clinical practice guidelines to collect additional eligible data. All electronic database searches were conducted from database inception to March 2024. Search terms were devised by the study team, which included a research librarian working in a third-level grade-A hospital, and subject headings were used where possible and adjusted for differences.
The following keyword combinations were used for the search: (Liver Transplantation OR Liver Transplant ∗ OR Hepatic Transplant OR Hepatic Grafting OR Liver Grafting) AND (Living donors OR donor ∗ OR donation) AND (qualitative research OR qualitative study OR qualitative descriptive OR qualitative method* OR qualitative methods OR qualitative analysis OR focus group OR interview OR attitude OR experience OR phenomenology OR feel* OR needs OR ground research). Only peer-reviewed qualitative studies relevant to the real feelings, inner needs, and emotional experiences of living liver donors were considered eligible for inclusion in this study. In this review, qualitative studies were defined as those using methodologies such as phenomenology, ethnography, grounded theory, hermeneutics, narrative and thematic analysis and/or studies reporting primarily textual rather than numerical analyses and findings.
Study selection
Following the search, all identified records were imported into Endnote X8 software (Clarivate Analytics, PA, USA), and duplicates were removed. Subsequently, two reviewers (L.D and Z.R.L) independently screened the titles and abstracts of the study to determine whether the study met the inclusion criteria. Full papers of abstracts were then reviewed regarding the inclusion and exclusion criteria. The inclusion criteria were as follows: (1) the samples were living liver donors (≥ 18 years); (2) the study focused on the real feelings, inner needs, and emotional experiences of living liver donors after surgery; (3) the study used a qualitative design; and (4) the study was published in English in peer-reviewed journals. Exclusion criteria were as follows: (1) unavailable full text or incomplete data in the literature; (2) repeated publication; and (3) studies including only clinicians’ views and experiences of caregivers on liver transplant. Additionally, the reasons for the exclusion of full-text papers were recorded. Discrepancies between the reviewers at each stage of the study selection process were resolved through discussion and assessed independently by a third reviewer in the research group when necessary.
Appraisal of methodological quality
Two reviewers (L.D and F.C.L) who had received systematic training in qualitative research independently evaluated the quality of the included studies using the 10-item Joanna Briggs Institute Critical Appraisal Checklist [ 16 ]. This checklist, used for evaluating the quality of reports on qualitative research outcomes, was selected because it was found to be the most coherent tool compared with other appraisal methods for qualitative research [ 17 ]. Disagreements between the reviewers regarding the risk of bias in the quality assessment of the included studies were resolved by discussion, and a third reviewer was involved in this process when necessary. A minimum of ‘yes’ for six domains was required for inclusion in previous studies [ 18 ].
Data extraction and synthesis
After confirming the eligibility of studies, the two reviewers independently extracted relevant data, including author, year of publication, aims or purpose of the study, donor-recipient type, sample, methodology, data collection and analysis, and themes. An arbitrator was consulted in cases of disagreement between the two reviewers.
We used Thomas and Hardens’ three-stage thematic synthesis approach [ 19 ]: (i) line-by-line coding of relevant texts; (ii) organization of codes into descriptive themes; and (iii) development of analytical themes. Microsoft Word was used to assist with the data synthesis. The first reviewer (L.D) carefully read the included studies to obtain an adequate understanding and coded the relevant extractions from these texts that captured the meaning of each sentence line by line. The codes were compared, consolidated and then grouped into descriptive themes. Finally, descriptive themes were developed and further interpreted to develop analytical themes. All coded data were re-examined by the first reviewer to ensure consistency and to determine whether additional coding was necessary. A second reviewer (Z.R.L) reviewed the codes, and disagreements were resolved through discussion throughout the process if necessary. Two reviewers compared the coded data and common themes across studies to establish concepts that pertained to more than one study, which can constitute a synthesis.
Rigor, trustworthiness, and reflexivity
Our study illuminated different themes by analyzing quotations from participants, not the authors’ themes or interpretations. The multidisciplinary team included academic nurses (role: aim development and interpretation), research assistants (role: data analysis, and synthesis), and a librarian (role: literature search) in a third-level grade-A hospital. L.D, was an MSc student and is interested in the experience of living liver donors. Z.R.L, is now a PhD student conducting research in the same area. F.Y.L and F.C.L have clinical and research experience relevant to the study topic. L.Z has research expertise in qualitative research and is currently engaged in research on symptom experience in liver transplant recipients. The nursing and research assistants were trained in the use of qualitative methods before conducting the study. Team members communicated regularly via WeChat meetings and face-to-face group discussions to conduct the meta-synthesis. Disagreements were resolved through discussion and, if necessary, evaluation by a third reviewer (L.Z). We presented our analytical themes to five people who had donated a liver to their family members and incorporated their suggestions into the final analytical themes.
Characteristics of the included studies
Based on the initial searches, 3880 articles were retrieved, and 3380 records remained after duplicates were removed. Subsequently, during the screening of titles and abstracts, 51 studies were selected for full-text screening. Following this, two authors independently read 51 full-text articles and excluded 42 of them. Ultimately, 9 studies that met the inclusion criteria were included. The results of the search are presented in line with the PRISMA guidelines, and flow charts are shown in Fig. 1 .

Flowchart of the study selection process
Among the included studies, two were conducted in the USA, two in Turkey, and one each in Canada, China, Germany, Japan, and Sweden. A total of 144 living liver donors participated in this study. Various qualitative methodologies were used, most commonly phenomenology [ 20 , 21 , 22 ]( n = 3); two studies [ 23 , 24 ] used grounded theory, one study [ 25 ]used ethnography, and 3 studies [ 26 , 27 , 28 ] did not report a specific approach, but identified the study as “qualitative”. Data were primarily gathered through interviews, and five studies reported data saturation. A broad range of data analysis methods were used, including content analysis, thematic analysis, interpretive phenomenological analysis, and grounded theory. Detailed information regarding the included studies is shown in Table 1 . Most studies met most Joanna Briggs Institute criteria, and the quality appraisal of the studies is reported in Table 2 .
Main findings of the synthesis
Through the meta-synthesis, four themes were identified: facing a life-changing situation, experiencing changes in interpersonal relationships, coping with changes, and achieving personal growth (Fig. 2 ). Review of analytical themes by our consultation with 5 living liver donors elucidated the importance for personal growth. Themes with key quotations are presented below.
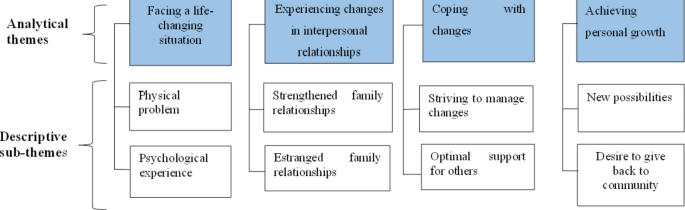
Synthesized themes and sub-themes
Theme 1: facing a life-changing situation
Donors described the donation as a life-changing event or, in some cases, as a life-saving gift [ 24 , 25 ]. When asked to provide further details regarding the specifics of these life-changing situations, most donors reported having experienced a period of significant physical and/or psychological distress. Facing a situation in which they were transformed from a healthy, strong, and suitable donor to someone with a recognized illness, donors reported experiencing physical impairments, such as bowel-related issues, the expected difficulties of movement and mobility, and severe pain [ 21 , 22 , 26 ].
“Someone at the liver surgery unit told me that after the operation there will be a slight pain in the wound. I have to say that was a gross understatement. I could never imagine that one could be so totally paralyzed by a wound.” [ 22 ].
Although donors perceived these physical impairments as necessary “tradeoffs” (e.g., the survival of the recipient versus physical impairments), psychological distress also became a cause of concern for them. In the included studies, living liver donors experienced feelings of loneliness, helplessness, vulnerability, hurt, and neglect after surgery and expressed a desire to receive care [ 22 , 27 ]; however, most health care providers and family members paid more attention to the recipient even after discharge from the hospital. Many donors continued to express strong concerns about the recipient, such as concerns regarding transplant complications, drug side effects, and test indicators [ 23 , 28 ].
“After surgery , my mother and I were put in the same room. No one showed interest in me. This disinterest continued after our discharge. I always had the role of an attendant , but I needed care too.” [ 27 ].
Despite the negative aspects mentioned above, the donors reported positive emotions. As the donation was viewed as a uniquely rewarding and meaningful experience, some donors described the pride, happiness, certainty, confidence and appreciation associated with being part of a specific group and being able to try their best to do good things for others [ 23 , 25 , 26 ]. Additionally, as the postoperative period progressed, their enjoyment of the donation improved due to the recipient’s recovery in terms of health.
“Um , and just kind of made me , definitely like a self-esteem boost knowing that I’m able to have the potential to do good things for other people.” [ 26 ].
Theme 2: experiencing changes in interpersonal relationships
In the included studies, donors exhibited both closer and estranged relationships with others who were close to them as a result of the donation. On the one hand, they explicitly indicated that they maintained an intimate, good or even better relationship with the recipient. They showed more understanding or interest in each other, had more trust in the relationship, and met or communicated more often; their relationship was thus described as a special and deepened bond [ 23 , 24 , 25 , 26 , 27 , 28 ]. Simultaneously, donors also noted the strengthening of their relationships with the family to some extent, which subsequently recreated their family and their inner self. Consequently, they viewed their donation as meaningful and rewarding.
“As a result of the illness , we started getting along very well with each other and better and better , till she could open herself to me and tell me everything which had happened to her , in her life. This made her feel better and we have become so honest with each other , that I could tell her my problems too.” [ 24 ].
“It creates a different bond between you , I mean , you donating your organ , your liver to your mother is an entirely different thing , it creates a completely different bond I mean.” [ 23 ].
On the other hand, donors experienced intense relationships, not only with the recipient but also with their families. For the former, due to feelings of indebtedness and gratitude towards their donors’ sacrifice, the recipient tended to put some distance between himself/herself and the donor. That might influence the authenticity of the communication, especially in the event of a conflict [ 24 , 27 ]. Regarding the latter, other family members believed that the donor valued the recipient more than the family relationship, and the donor did not receive sufficient support from family members during the donation process, which led to an estranged family relationship [ 25 , 27 ].
“Everything got worse after the transplantation. He said that I treated him badly since he owed me. However , I didn’t do anything bad to him. He misunderstood me.” [ 27 ].
“I knew it was something that bothered her a lot, and she was angry at me about it. [Laughs] It was resentment that moved toward anger later on. It expressed itself, you know, in ways that I had never imagined.” [ 25 ].
Theme 3: coping with changes
The considerable impact on the physical and psychological health of donors necessitates appropriate coping strategies to address the effects of the situation. Several studies have reported that many donors strive to adapt, accept, or surrender to this life-changing situation after surgery [ 20 , 21 , 24 , 28 ]. Specific examples included striving to control negative emotions, viewing complications as reversible and manageable, removing themselves from information, exhibiting overall confidence, and assigning value to donation [ 20 , 21 , 24 , 28 ]. Other donors also mentioned that religion and beliefs are significant in helping them cope with the situation and experience less stress [ 23 ].
“I take care of myself very well. I think it [liver donation] was not a problem. I only needed to donate a part of liver. The liver will regenerate , and I will recover after surgery quickly.” [ 20 ].
“Thank you God for granting me such thing so that I can do something good for my child. You gave me such a good liver that it also did good to her.” [ 23 ].
In addition, most donors noted that support from family, friends, medical staff and the community was integral to the task of coping with the most difficult times following their donation [ 23 , 24 , 25 ]. These sources provided financial support and emotional support by discussing the donation process, providing disease-related information, offering verbal words of encouragement and praise, helping the donors manage their emotions, praying with the donors and celebrating the donors’ actions, which made the donors feel loved, motivated, relieved and supported.
“Very often and we write to each other. We are in contact at least two or three times a week , if possible. And then I also helped her financially. She’s living from welfare , so I’d like to support her financially. And I am writing really nice letters to her , she is replying and we talk and she encourages me on the phone.” [ 24 ].
“If I didn’t understand something , we were going to discuss it and make sure that I did understand what was going on. Dr C was very thorough , he’d get a piece of paper or a napkin , and he would draw me a picture and show me exactly what’s connecting and where it’s going , and he really did put the information out there so that you could ask the questions.” [ 25 ].
However, other donors noted that the information and emotional support provided by family and medical staff were less than optimal [ 22 , 23 , 27 ].
“My father and mother are very old , they are in the village. When I had this operation , they weren’t here.” [ 23 ].
“Little or no support was provided. You get the feeling that such resources are not prioritised at all… Yes , there is a great deal of fear and thoughts and stuff that you can’t share with others , there’s just no time for it.” [ 22 ].
Theme 4: achieving personal growth
Finally, a theme identified in some included studies focused on participants’ descriptions of personal growth. While these studies depicted fairly dire circumstances with regard to physical recovery and the psychological struggles associated with donation, these struggles appeared to be the source of growth and meaning for many donors. Some donors noted that their previous understanding of their personal limitations and abilities was shattered and gained confidence and new perceptions regarding their capabilities [ 25 ], which enabled them to accomplish things that previously seemed to be unrealistic or unachievable and to take a step forward for a new path in their lives [ 26 , 28 ], for instance, starting to perform in front of large audiences, which they had never thought possible before.
“And I can just keep growing in any way that I want to now , and I don’t have to , like , listen to the no person in your head that , like , just like , ‘No , you can’t do that.’ Why not? Why the heck not? You know? Like , you defy the things you think are possible , and then all of a sudden everything becomes possible.” [ 26 ].
More importantly, for some donors, as a result of their newly acquired perspectives following donation, they strongly desired to give something back to the society from which they had received so much [ 25 , 26 ]. One donor stated that “The donation did open our eyes to do something to reach out and to pick your neighbour up, and we knew right after the donation that we were now responsible for putting something back into the kitty” [ 25 ]. In addition, the donation experience led donors to change their outlook and perspective on life; they learned to enjoy life now instead of just focusing on furthering their careers or their children’s performance at school [ 21 , 23 , 28 ].
“It made me realize who I am and what I want to be , the value of life. That you take things for granted or you can just let life pass you by and not do anything.” [ 21 ].
“It’s provided me a more , um , awareness [… .] and consciousness of my place in society , in my community , my spiritual and physical connection , and my desire to just continue on the same ethos of helping people where possible.” [ 26 ].
This review identified, compared and synthesized nine qualitative articles with the aim of exploring the lived experiences of living liver donors. Four themes emerged: facing a life-changing situation, experiencing changes in interpersonal relationships, coping with changes and achieving personal growth.
First, the theme of facing a life-changing situation showed that living liver donors experienced physical trauma and psychological changes following surgery. Most donors described a variety of physical changes after surgery in the studies included in this review. This finding supports a previously reported systematic review [ 7 ] that included 13 prospective longitudinal studies and concluded that living liver donors reported decreased physical functioning after surgery that were returning to pre-donation levels by two years post-donation. A study [ 29 ] with the longest mean follow-up period of 11.5 years revealed that living liver donors continue to maintain excellent quality of life outcomes up to 20 years after donation and return to their normal daily life without any reported lasting physical or psychosocial concerns. In addition, psychological changes have also been observed in living liver donors after surgery. For example, donors not only report experiencing feelings of loneliness, helplessness, vulnerability, hurt and neglect and great concern for the recipient after surgery, but also report positive emotions such as pride, joy, certainty, confidence and gratitude. This finding is consistent with Kisch et al.’s [ 30 ] review in 2018. Negativity causes donors to feel vulnerable and adversely affects donor access to mental health, while worry about recipients, lack of psychological support, and uncertainty about the future often exacerbate donor psychological distress. Positive psychology posits that positive psychological traits are a manifestation of psychological defence, which can help individuals develop positive psychological coping strategies. Studies [ 31 , 32 ] have shown that positive emotions are adaptive and can enhance an individual’s psychological function and social connection, increase well-being, reduce physical and mental health risks, and ultimately help donors adapt to recovery more effectively. The combined results of this study indicated that donor-reported positive psychological changes overlapped with published finding [ 33 , 34 ]. For example, many donors feel increased self-esteem and self-affirmation and exhibit positive lifestyle changes. As discussed in the study conducted by Rudow et al. in 2014 [ 31 ], the donors after liver transplantation had greater life expectation. Therefore, from the perspective of positive psychology, the transplant team, including nurses and coordinators, should fully understand the donor’s psychological experience, pay attention to the positive role of psychological traits, take into account the personality traits of living donors and recipient’s disease stage, and jointly explore the supporting factors of positive psychological experience in the living liver donor, ensuring that the donor’s positive psychological state can be well adapted and maintained to the greatest extent and the influence of the negative emotions of the donor can be reduced, especially in cases of poor donor or recipient outcomes.
Subsequently, we observed changes in the interpersonal relationships experienced by donors as part of their lived experience. At least 6 of the 11 studies we reviewed included descriptions of relationship changes (e.g., receiver related, partner related and integral family related). Similarly, the “donor-recipient relationship after living kidney donation” emerged as the main issue in a recent systematic review [ 35 ], which focused specifically on the psychological impact of living kidney donation on donors. This review [ 35 ] and other studies [ 36 , 37 , 38 ] have shown that relationships among donors and recipients and their families rarely deteriorate after transplant; in fact, they often remain the same or even improve. Assessing the relationship between the recipient and donor is a key component of the psycho-social assessment of the transplant process. Facilitating access to post-transplant psychological support with the aim of addressing potentially deteriorating relationship changes may help living donors adjust to changes in relationship dynamics more effectively, which in turn may contribute to improving their psycho-social and transplant outcomes [ 39 ]. Gift-exchange theory [ 40 ] provides a logical explanation for changes in the donor-recipient relationship, and this understanding should help the transplant team assist donors, recipients, and their families throughout the process.
The integrated results of this study suggest that positive personal responses and support from multiple sources can help donors cope with donation more effectively. Only by establishing and maintaining a good support system for living liver donors in an all-around way with the aim of meeting their support needs can the physical and mental health of living liver donors be effectively achieved and maintained [ 41 , 42 , 43 , 44 ]. Therefore, targeted support should be given based on the specific circumstances of the donor in question. In terms of information support, the transplantation team should provide the donor with sufficient information about the operation to help the living liver donor understand the overall process, procedures and risks associated with donation [ 43 ]. Simultaneously, to alleviate the financial difficulties of donors, it is necessary to expand the public burden of financial assistance and expenses and to increase financial assistance throughout the entire donation process, including donor suitability checks during the donation process, surgery and nursing care, and post-donation health checks [ 45 ]. At the family level, transplant families should be encouraged to participate in the procedure as much as possible, strengthen care for donors, to promote emotional communication with transplant donors, establish a multidimensional support model, reduce the psychological burden of donors, and encourage donors to respond more actively to life after donation [ 46 ]. With the multidisciplinary cooperation of medical staff, social workers and transplant coordinators, liver donors can be provided with continuous post-donation social adjustment counselling and emotional support to help them return to normal and maintain their quality of life [ 47 ].
The theme of “achieving personal growth” showed that living liver donors undergo a transformative journey encompassing both physical recuperation and psychological challenges, culminating in positive personal growth. Living donation implies reciprocity, as the living donor donates a fragment of their liver with the intention of giving life or health to another person. In return, the donor feels that his or her life is given greater meaning, leading to a profound appreciation of life. Some liver donors even compared the donation experience to the greatness of giving birth, reflecting the giving of life in different ways [ 16 ]. This finding was consistent with a recent review [ 48 ], which demonstrated that living kidney donors gained benefits from the donation experience. Despite the relative absence of discussions in the earlier review [ 30 , 49 ] related to the positive personal growth of living donors, our findings supported the results of other studies [ 50 , 51 ]. Other studies have found that the act of donation appears to be a catalyst for positive long term personal growth. These findings help to bolster the ethical argument in favour of living liver donation. In addition, it’s important to recognize that the lived experience of living liver donors may vary depending on the relationship between the donor and the recipient. However, comparisons to elucidate the differences in experiences between directed liver donors and nondirected donors are limited due to the sparsity of data on anonymous liver donors. In the case of directed liver donors, the majority of these individuals are from first-degree relatives, spouses, or partners [ 52 ]. These donors frequently describe their donation as a rebirth of a loved one [ 53 ]. Nondirected donors, who donate organs to someone in need, tend to view their donation as a gift exchange, giving and receiving a gift-reciprocity [ 54 ].
Implications
This study systematically reviewed and analyzed the lived experiences of living liver donors after surgery, and its results can help transplant teams obtain comprehensive insights into the inner world of living liver donors and could inform tailored care. Specifically, transplant teams should pay more attention to donors’ physical, psychological, and social changes after surgery, closely monitor the physical problems of liver donors in the short-term, and provide ongoing psychological counselling and family support to help living liver donors return to the normal life. Especially those for donors or recipients with poor outcomes should be treated as a vulnerable group requiring increased attention and continued follow-up after donation. More importantly, this study provides an understanding of positive personal growth in living liver donors. Living donors who have had positive experiences help potential donors gain a deeper understanding of the donation process by sharing their personal experiences and providing emotional support, empowering them to make informed and confident decisions. Future nursing research should focus on the positive aspects of living liver donors and explore how these positive aspects can be facilitated and maximised to help living liver donors achieve their optimal level of functioning.
Limitations
However, some limitations of this study should also be taken into account. Firstly, despite the fact that this search was comprehensive and systematic, some studies may have been missed; in addition, we included only papers published in English, and some non-English papers and grey literature may have been missed. Secondly, we included studies on the lived experience of donors between paediatric liver transplantation and adult liver transplantation, which may have influenced the integration results to some degree.
This meta-synthesis of qualitative studies explored the physical, psychological, and social changes that occur in living liver donors and highlighted the importance of coping strategies and gaining personal growth. This review provides a more comprehensive experience of living donors than is currently in the literature. Promoting the early recovery of living liver donors and improving their quality of life are essential for healthcare providers. Identifying current coping strategies will allow nurses and living liver donors to collectively assess the effectiveness of these strategies and facilitate adaptation when more effective responses are needed. Finally, future studies need rigorous research methods, clear methodological approaches, and consider applying or developing theories to advance academic research and patient-centered interventions for living liver donors.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, Schnellinger EM, Handarova D, Weiss S, Cafarella M, et al. Liver Am J Transpl. 2023;23(2):S178–263. OPTN/SRTR 2021 Annual Data Report.
Terrault NA, Francoz C, Berenguer M, Charlton M, Heimbach J, Liver. Transplantation 2023: Status Report, Current and Future Challenges. Clin Gastroenterol Hepatol. 2023;21(8):2150-66.
Sakai T, Ko JS, Crouch CE, Kumar S, Little MB, Chae MS, Ganoza A, Gómez-Salinas L, Humar A, Kim SH, et al. Perioperative management of adult living donor liver transplantation: Part 1 – recipients. Clin Transpl. 2022;36(6):e14667.
Article Google Scholar
Sakai T, Ko JS, Crouch CE, Kumar S, Choi GS, Hackl F, Han DH, Kaufman M, Kim SH, Luzzi C, et al. Perioperative management of living donor liver transplantation: Part 2 - Donors. Clin Transpl. 2022;36(6):e14690.
Jeong SJ, Kim HN. Experiences of physical complications and sequelae among living liver donors. Korean J Transpl. 2019;33(2):36–45.
Thuluvath AJ, Peipert J, Berkowitz R, Siddiqui O, Whitehead B, Thomas A, Levitsky J, Caicedo-Ramirez J, Ladner DP. Donor quality of life after living donor liver transplantation: a review of the literature. Dig Med Res. 2021;4:49.
Article PubMed PubMed Central Google Scholar
Shi Y, Liu C, Zhang H, Huang Y, Sun M, Wang W, Shang S. Changes in the quality of life of living liver donors: A meta-analysis. Int J Nurs Stud. 2020;109:103586.
Article PubMed Google Scholar
Shen CJ, Huang HL, Chen KH, Weng LC, Wang SY, Lee WC, Chou HF, Tsai HH. Comparison of Liver Function, Emotional Status, and Quality of Life of Living Liver Donors in Taiwan. Transpl Proc. 2016;48(4):1007–11.
Ispir M, Cumhur B, Sahin T, Otan E, Kayaalp C, Yilmaz S. Psychosocial Outcomes of Donors Whose Recipients Died After Living Donor Liver Transplantation. J Gastrointest Cancer. 2020;51(4):1200–08.
Ryu S, Yoon SC, Hong KE, Kim JM. Psychosocial Issues Related to Donor’s Decision-Making in Living Donor Liver Transplantation. Ann Transpl. 2019;24:576–83.
DiMartini A, Dew MA, Liu Q, Simpson MA, Ladner DP, Smith AR, Zee J, Abbey S, Gillespie BW, Weinrieb R, et al. Social and Financial Outcomes of Living Liver Donation: A Prospective Investigation Within the Adult-to-Adult Living Donor Liver Transplantation Cohort Study 2 (A2ALL-2). Am J Transpl. 2017;17(4):1081–96.
Article CAS Google Scholar
Kaplan A, Aby ES, Scott S, Sonnenday C, Fox A, Mathur A, Olthoff K, Heimbach J, Ladin K, Emamaullee J. Financial toxicity in living donor liver transplantation: a call to action for financial neutrality. Am J Transpl. 2024. https://doi.org/10.1016/j.ajt.2024.05.012 .
Sarıgöl Ordin Y, Harmancı Seren AK, Karayurt Ö, Aksu Kul G, Kılıç M, Bozoklar CA, Tokat Y. Evaluation of Psychosocial Outcomes of Living Liver Donors in Liver Transplantation. Turk J Gastroenterol. 2022;33(4):346–55.
Raza MH, Jackson WE, Dell A, Ding L, Shapiro J, Pomfret EA, Genyk Y, Sher L, Emamaullee J. Health-related quality of life after anonymous nondirected living liver donation: A multicenter collaboration. Am J Transpl. 2021;21(3):1056–67.
Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181.
The Joanna Briggs Institute. The Joanna Briggs Institute Critical Appraisal tools for use in JBl. Systematic Reviews: Checklist for Oualitative Research; 2017.
Google Scholar
Hannes K, Lockwood C, Pearson A. A comparative analysis of three online appraisal instruments’ ability to assess validity in qualitative research. Qual Health Res. 2010;20(12):1736–43.
Boehm LM, Jones AC, Selim AA, Virdun C, Garrard CF, Walden RL, Wesley Ely E, Hosie A. Delirium-related distress in the ICU: A qualitative meta-synthesis of patient and family perspectives and experiences. Int J Nurs Stud. 2021;122:104030.
Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8:45.
Weng L, Huang H, Wang Y, Chang C, Tsai C, Lee W. The Coping Experience of Taiwanese Male Donors in Living Donor Liver Transplantation. Nurs Res. 2012;61(2):133–39.
Charlotte C, Cabello, Smolowitz J. Roller coaster marathon: being a live liver donor. Prog Transpl. 2008;18(3):185–91.
Forsberg A, Nilsson M, Krantz M, Olausson M. The essence of living parental liver donation–donors’ lived experiences of donation to their children. Pediatr Transpl. 2004;8(4):372–80.
Krespi MR, Tankurt A, Acarlı K, Yankol Y, Kalayoglu M, Kanmaz T, Duregger C. Post-donation evaluation of life of donors of liver transplantation. Cogent Psychol. 2016;3(1):1262724.
Papachristou C, Walter M, Schmid G, Frommer J, Klapp BF. Living donor liver transplantation and its effect on the donor-recipient relationship–a qualitative interview study with donors. Clin Transpl. 2009;23(3):382–91.
Nasr AS, Rehm RS. Parental live liver donation: a transformational experience. Prog Transpl. 2014;24(1):69–75.
Krause S, Pritlove C, Abbey S, Jung J. Growth through adversity: posttraumatic growth in anonymous living liver donors. Disabil Rehabil. 2022;44(8):1328–32.
Ordin YS, Karayurt O, Aksu Kul G, Kilic M, Taylor LA. Exploration Into Donor-Recipient Relationship After Living-Donor Liver Transplantation Using Gift-Exchange Theory. Florence Nightingale J Nurs. 2021;29(2):150–57.
Tomoko Kusakabe S, Irie N, Ito, Kazuma K. Feelings of living donors about adult-to-adult living donor liver transplantation. Gastroenterol Nurs. 2008;31(4):263–72.
Raza MH, Kim MH, Ding L, Fong TL, Romero C, Genyk Y, Sher L, Emamaullee J. Long-Term Financial, Psychosocial, and Overall Health-Related Quality of Life After Living Liver Donation. J Surg Res. 2020;253:41–52.
Kisch AM, Forsberg A, Fridh I, Almgren M, Lundmark M, Lovén C, Flodén A, Nilsson M, Karlsson V, Lennerling A. The Meaning of Being a Living Kidney, Liver, or Stem Cell Donor-A Meta-Ethnography. Transplantation. 2018;102(5):744–56.
Rudow DL, Iacoviello BM, Charney D. Resilience and personality traits among living liver and kidney donors. Prog Transpl. 2014;24(1):82–90.
Duan W, Guo P, Gan P. Relationships among Trait Resilience, Virtues, Post-traumatic Stress Disorder, and Post-traumatic Growth. PLoS ONE. 2015;10(5):e0125707.
Thys K, Schwering KL, Siebelink M, Dobbels F, Borry P, Schotsmans P, Aujoulat I. Psychosocial impact of pediatric living-donor kidney and liver transplantation on recipients, donors, and the family: a systematic review. Transpl Int. 2015;28(3):270–80.
Butt Z, Dew MA, Liu Q, Simpson MA, Smith AR, Zee J, Gillespie BW, Abbey SE, Ladner DP, Weinrieb R, et al. Psychological Outcomes of Living Liver Donors From a Multicenter Prospective Study: Results From the Adult-to-Adult Living Donor Liver Transplantation Cohort Study2 (A2ALL-2). Am J Transpl. 2017;17(5):1267–77.
Cazauvieilh V, Moal V, Prudhomme T, Pecoraro A, Piana A, Campi R, Hevia V, Territo A, Boissier R. Psychological Impact of Living Kidney Donation: A Systematic Review by the EAU-YAU Kidney Transplant Working Group. Transpl Int. 2023;36:11827.
Giessing M, Reuter S, Schönberger B, Deger S, Tuerk I, Hirte I, Budde K, Fritsche L, Morgera S, Neumayer HH, et al. Quality of life of living kidney donors in Germany: a survey with the Validated Short Form-36 and Giessen Subjective Complaints List-24 questionnaires. Transplantation. 2004;78(6):864–72.
Ralph AF, Butow P, Craig JC, Wong G, Chadban SJ, Luxton G, Gutman T, Hanson CS, Ju A, Tong A. Living kidney donor and recipient perspectives on their relationship: longitudinal semi-structured interviews. BMJ open. 2019;9(4):e026629.
Agerskov H, Bistrup C, Ludvigsen MS, Pedersen BD. Experiences of living kidney donors during the donation process. J Ren Care. 2018;44(2):96–105.
Ralph AF, Butow P, Hanson CS, Chadban SJ, Chapman JR, Craig JC, Kanellis J, Luxton G, Tong A. Donor and Recipient Views on Their Relationship in Living Kidney Donation: Thematic Synthesis of Qualitative Studies. Am J Kidney Dis. 2017;69(5):602–16.
Gill P, Lowes L. Gift exchange and organ donation: donor and recipient experiences of live related kidney transplantation. Int J Nurs Stud. 2008;45(11):1607–17.
Li L, Yang L, Dong CQ, Yang QL, Huang WJ, Liao TT, Sun XH. Psychosocial Assessment of Donors in Pediatric Living Donor Liver Transplantation: A Systematic Review. Transplant Proc. 2021;53(1):3–15.
Gökçe S, Durmaz O, Peykerlı Gürsu G, Aydoğan A, Celtık C, Ozden I, Sökücü S. Assessment of living donors with respect to pre- and posttransplant psychosocial properties and posttransplant family functioning in pediatric liver transplantation. Turk J Gastroenterol. 2011;22(1):36–41.
Goldschmidt I, Migal K, Rückert N, van Dick R, Pfister ED, Becker T, Richter N, Lehner F, Baumann U. Personal decision-making processes for living related liver transplantation in children. Liver transpl. 2015;21(2):195–203.
Lai YC, Lee WC, Juang YY, Yen LL, Weng LC, Chou HF. Effect of social support and donation-related concerns on ambivalence of living liver donor candidates. Liver transpl. 2014;20(11):1365–71.
Emamaullee J, Tenorio L, Khan S, Butler C, Kim S, Tucker-Seeley R, Kwon Y, Shapiro J, Saigal S, Sher L, et al. Living donor financial assistance programs in liver transplantation: The global perspective. Clin Transpl. 2020;34(11):e14073.
Lim WH, Chan KE, Ng CH, Tan DJH, Tay PWL, Chin YH, Yong JN, Xiao J, Fu CE, Nah B, et al. A qualitative systematic review of anonymous/unspecified living kidney and liver donors’ perspectives. PLoS ONE. 2022;17(12):e0277792.
Article CAS PubMed PubMed Central Google Scholar
Adams MA, Andacoglu O, Crouch CE, de Santibañes M, Jackson WE, Jalal A, Montasser IF, Rubman S, Spiro M, Raptis DA, et al. Does pre-operative counselling of the donor improve immediate and short-term outcomes after living liver donation? - A review of the literature and expert panel recommendations. Clin Transpl. 2022;36(10):e14636.
Massey EK, Rule AD, Matas AJ. Living Kidney Donation: A Narrative Review of Mid- and Long-term Psychosocial Outcomes. Transplantation. 2024. https://doi.org/10.1097/TP.0000000000005094 .
Tong A, Chapman JR, Wong G, Kanellis J, McCarthy G, Craig JC. The motivations and experiences of living kidney donors: a thematic synthesis. Am J Kidney Dis. 2012;60(1):15–26.
Al Breizat AH, Abunaser MT, Al Breizat Z. Living Donors: Altruism and Feeling Forgotten. Exp Clin Transpl. 2020;18(Suppl 1):22–8.
Yucetin L, Bozoklar CA, Yanik O, Tekin S, Tuncer M, Demirbas A. An Investigation of Post-Traumatic Growth Experiences Among Living Kidney Donors. Transpl Proc. 2015;47(5):1287–90.
Raza MH, Aziz H, Kaur N, Lo M, Sher L, Genyk Y, Emamaullee J. Global experience and perspective on anonymous nondirected live donation in living donor liver transplantation. Clin Transpl. 2020;34(4):e13836.
Bahador RS, Farokhzadian J, Mangolian P, Nouhi E. Concerns and Challenges of Living Donors When Making Decisions on Organ Donation: A Qualitative Study. Iran J Nurs Midwifery Res. 2022;27(2):149–56.
Bailey PK, Ben-Shlomo Y, de Salis I, Tomson C, Owen-Smith A. Better the donor you know? A qualitative study of renal patients’ views on ‘altruistic’ live-donor kidney transplantation. Soc Sci Med. 2016;150:104–11.
Download references
Acknowledgements
The authors would like to thank all authors of the included original studies, and all participants in this study for their friendly cooperation.
Author information
Authors and affiliations.
Department of Nursing, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Li Dong, Zhiru Li, Li Zheng & Fangyan Lu
School of Nursing, Zhejiang University School of Medicine, Hangzhou, China
Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, NO.79 Qingchun Road, Hangzhou, Zhejiang Province, China
Feicui Lv & Fangyan Lu
You can also search for this author in PubMed Google Scholar
Contributions
F.Y.L designed the study; L.D and Z.R.L. conducted screening, selecting, and synthesis relevant articles; F.C.L and L.D. evaluate the quality of the evidence; L.D wrote the manuscript; F.Y.L and L.Z. reviewed and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Fangyan Lu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Dong, L., Li, Z., Lv, F. et al. The lived experience of living liver donors: A meta-synthesis of qualitative studies. BMC Nurs 23 , 925 (2024). https://doi.org/10.1186/s12912-024-02611-7
Download citation
Received : 12 April 2024
Accepted : 11 December 2024
Published : 19 December 2024
DOI : https://doi.org/10.1186/s12912-024-02611-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liver transplant
- Living liver donor
- Meta-synthesis
- Qualitative research
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]

IMAGES
COMMENTS
Organ donation is defined as giving an organ or part of an organ to be transplanted into another person. Organ transplantation is the only option to save lives in patients affected by terminal organ failures and improve their quality of life. However, there is a disparity exists between the supply and demand of donated organs, leads to a loss of many lives. The number of organ transplantation ...
Organ donation is defined as giving an organ or part of an organ to be transplanted into another person. Organ transplantation is the only option to save lives in patients affected by terminal organ failures and improve their quality of life.
Get a tailor-made essay on 'Why Organ Donation after Death Should Be Encouraged' ... We can provide someone the chance to spend more time with their family or allow a child to live a full life. Encouraging organ donation can make these possibilities a reality (Green, 2021). ... 2021). Cultural Perspectives and Ethical Considerations. In Peru ...
Because there are already too few brain-dead donors - each year, more than 8,000 people in the US die while on the waiting list for organs - medicine has tapped a new source of organs in the form of donation after circulatory death (DCD). These organ donors are not brain dead, although many of them are unconscious. Instead, they lack ...
The Organ donation (OD) is defined as the process of retrieving or procuring an organ or part of it from a live or deceased person and then, transplanting it into another living person. 1 It has ...
The procedure combining medical assistance in dying (MAiD) with donations after circulatory determination of death (DCDD) is known as organ donation after euthanasia (ODE). ... The databases were queried for English language papers published between 2017 and 2021 relating to the current practice of MAiD and DCDD where relevant to ODE and for ...
In 2020, DCD donors accounted for 25.6% of all deceased organ donors in the U.S, 1 and there was an 18.6% increase in the number of DCD donors in 2020 compared to 2019. 2 Donation after brain death (DBD) makes up the rest of the deceased donor pool in the US but has less potential for a substantial increase in numbers, as only about 2% of ...
However, despite its critical importance, there persists a significant gap in awareness and understanding surrounding organ donation. This essay embarks on a journey to shed light on the multifaceted dimensions of organ donation, encompassing its types, benefits, and the misconceptions that often shroud this life-saving practice.
The Life-Saving Benefits of Organ Donation Organ donation isn't just an act of kindness—it's huge! One donor can actually save up to eight lives by donating different organs like the heart, liver, kidneys, lungs, pancreas, and intestines.
Background Living liver donors are frequently used for liver transplantation, but they experience many physical changes and psychological challenges. A better understanding of the experiences of liver donors can provide them with comprehensive and individualized patient-centred support and health care. Thus, this study sought to identify and synthesize existing qualitative studies to develop ...