- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School

Mixtures for Kids
September 2, 2020 By Emma Vanstone 5 Comments
What is a Mixture?
A mixture is a substance in which two or more substances are mixed but not chemically joined together, meaning that a chemical reaction has not taken place.
Mixtures can be easily separated and the substances in the mixture keep their original properties.
Imagine mixing skittles and full size marshmallows, the individual components (skittles and marshmallows) could easily be separated using a filter and each component of the mixture ( skittles and marshmallow ) doesn’t change.
How to make a mixture
You can make your own mixtures with items from around the house.
1. Firstly try to make a mixture of toys.

2. This time use cereals or sweets.

What is a solution?
A solution is made when a solid (which we call a solute) dissolves into a liquid (that we call the solvent) One example of a solution is salt dissolved in water. The salt and water can be separated again by evaporation ( the water will evaporate if left in a hot place leaving he salt behind ).
Investigation
Aim : To test out these three mixtures to see which form solutions and which don’t
- Salt and Water
- Sugar and Water
- Sand and Water
Results Table
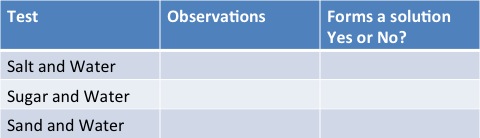
You should find that both salt and water and sugar and water dissolve and form solutions and that sand sinks to bottom!
How do you separate mixtures?
Can you separate the components out of the mixture again? Hint – to separate the sand from water you could use a sieve. This is possible as the sand is insoluble ( doesn’t dissolve in water ).
Salt and sugar are soluble ( dissolve in water ) and can be separated by evaporation.
Another way to separate a mixture is by using a process called chromatography .
Challenge – how would you separate rock salt and water?
Rock salt is a mixture of salt and sand and is often spread on roads in winter to stop cars skidding.
Stage 1 – Grinding
First the rock salt should be ground using a pestle and mortar.
Stage 2- Dissolving
The ground rock salt should be dissolved in a beaker and stirred thoroughly.
Stage 3 – Filtering
The solution of water and rock salt should be passed through the filter paper where the sand ( which will not have dissolved in the water ) will collect.
Salt does dissolve in water and so will pass through the filter paper.
Stage 4 – Evaporating
To separate the salt from the water the water needs to be evaporated off, either by leaving the salty solution in the sunshine or placing under a heat source.
The salt will form as crystals – this process is called crystallisation .

Last Updated on May 24, 2021 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
July 18, 2012 at 2:17 pm
Those are great educational play activities. I love your ideas for teaching the difference between a mixture and a solution in a meaningful way. Families can try out your mixtures and solutions and then come up with their own too. Thank you for sharing this on Artsy Play Wednesday on Capri + 3.
: 0 ) Theresa
July 23, 2012 at 6:50 am
Thank you. I’m glad you like it. x
October 08, 2020 at 12:08 pm
This helps me to keep my child busy and I love it So well done!
February 24, 2021 at 8:44 pm
Simple, fun, and accurate!! Thanks from myself and my e-schooled granddaughter 😉
October 08, 2021 at 3:29 pm
I´m a middle school student and and it has helped me understand things better
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

Separation of Mixtures Lab Activities for High School Chemistry

We went over recently that I don’t love the separation of mixtures lesson because it’s just so vocabulary heavy. After finding a way to get my students more engaged in that lesson, it came time to get them engaged in the separation of mixtures labs. Now don’t get me wrong, your girl LOVES a good distillation. But it’s near impossible to be able to do that in a standard high school chemistry lab. I’ve used and loved both of these separation of mixtures labs in my high school chemistry classroom and honestly, they’re not even that hard to do! Plus there’s a bonus in here, you may not have even realized was a separation of mixtures lab!
To preface, I teach 6 methods for separation of mixtures : distillation, evaporation, chromatography, precipitation reaction, separatory funnel and filtration.
Chromatography Lab
For this separation of mixtures lab, you’ll only need a few materials. If you don’t have what you need, you may be able to borrow from the art teacher in your school. You’ll need water, beakers, pencils or stirring rods, tape, coffee filters (or nice funnel filters) and washable markers (they need to be water soluble).
Student Procedure
Fold the coffee filter into a pizza slice to find the center. Unfold it and pop a tiny hole in the center. Put a rolled up piece of filter paper through this hole to make an umbrella out of the two filter papers. On the round piece of filter paper dot the different colors of ink in a circle around your pinhole. The circle should have a half inch radius. Put about a half inch of water into the beaker. Drop the “umbrella handle” into the water and let the water climb up the paper to the ink dots. As the water reaches the ink, it will spread. The more polar the ink, the more it will spread on the paper. (It clings to the water molecules and hitches a ride. Nonpolar inks won’t move as much).
Sequence of the Chemistry Course
I’ve written about this a few times before, but this lab is the reason I teach the Matter Unit so late in the year. Students need to know the concept of molecule polarity before they can truly understand how chromatography works. In my first year of teaching, I hadn’t put this together yet, and taught Matter really early in the year. To my embarrassment, I had to teach chromatography as “separation of mixtures based on differences in “molecular magnetism.” And then had to go back and change my language. If you need help organizing your chemistry course, check out my free chemistry curriculum outline. It’ll give you the reasoning as to why I teach what I teach in order, plus give you a day-by-day look at my chemistry course.

Evaporation Lab: Separation of Sand and Salt
This one is a lot of fun, and was born out of a pretty common state final exam question. “How should a student separate a mixture of sand and salt?” Well instead of hypothetically doing it, I decided we should do it for real.
Teacher Set Up
All I need to do is measure out sand and salt samples. I like to do some variety in my samples so students can’t just copy from each other. Also, my tip is to make a few extra samples in case your student spills the sample before even making it to their lab bench… (been there, done that). Obviously, make a note of the amount of sand and salt in each sample. I wouldn’t go beyond 5 grams total, but 3 is a pretty good sweet spot.
Students will measure the full mass of the sample, then drop it in water to dissolve the salt. It’s best to use as little water as possible, because they’ll need to evaporate the water in order to get the salt back. Then the wet sample goes into a funnel with filter paper. The sand will be caught but the salt water goes through. The sand should be washed with water to make sure all the salt becomes part of the filtrate. The sand can be put in an oven or set on a counter for a day or so to dry out. Just make sure it’s not near your air vents! This could also be warmed on a hot plate if you need it to be quicker!
The water will need to be evaporated from the salt. This requires a hot plate, or a Bunsen burner set up. I prefer the hot plate simply because the Bunsen burners can be a pain and need a LOT more monitoring than the hot plate. Use what you have, and what you feel comfortable with!
Students then measure how much sand, and how much salt they recovered. With that sum, they do a percent error calculation based on the original sample size. If you choose to give them the data, they can do a percent error for each component of the mixture as well.

Reasons I Love this Lab
I love this lab for a few reasons. One – It feels like a college level lab, with a lot of moving parts, data collection and calculations . But it’s certainly within the capacity of a high school student. Two – It showcases some great lab techniques : evaporation, funnel set up, washing a sample, drying a sample, and precise data collection. Three – the students feel like scientists when they do it. I’m a big fan of the fun labs, like making ice cream and slime, but those make kids feel like kids. Some labs are inherently “science-y” and this is one of them. Four – it’s a good real lab situation that I know my students will ace on that final exam because they’ve done the whole thing. Five – it’s great prep work for solubility rules , which based on my chemistry curriculum outline , is just a few weeks away.
BONUS: Dehydration of a Hydrated Salt
In this lab, my students are removing water from a hydrated salt. I prefer copper (II) sulfate pentahydrate because I love the color change! While I prefer to use this lab in my Stoichiometry Unit when learning about percent composition , it’s also a good separation of mixtures lab. If you weren’t able to get to it during your Stoichiometry Unit, now is a great time to use it! Check it out inside my full year lab manual .
You can find all of these separation of mixtures labs in my full year chemistry lab manual . You can also find them individually in my TPT store.
Share this:
Related posts.

Fun Experiments For Separating Mixtures

Chances are that you separate mixtures often. For example, any time you separate laundry or pick a topping off a pizza or drain a batch of freshly cooked pasta, you are separating a mixture. A mixture is a combination of substances that do not react chemically when they are mixed. According to this definition, a solution — such as sugar water — is a mixture just the same as a mixture of sugar and sand.
Fun Filtering
In this experiment you will observe how rudimentary separating techniques are better suited for certain mixtures, while others require slightly higher technology. Mix uncooked rice, kidney beans and flour in a mixing bowl. Spread the mixture onto a large sheet of wax paper, and you'll notice that the beans are easy to see. Pick them out by hand and place them in a cup. Separating the rice from the flour, however, won't be so easy. Prepare a sieve by cutting out a square section of window screen large enough to fit over the bowl. Set the screen over the mouth of the bowl, and affix it with a large rubber band. Gather the wax paper into a funnel shape, and slowly pour the flour and rice mixture onto the screen. The flour will pass through, leaving the rice on top.

Attracting Opposites
Separating a mixture of identical solids can be challenging until you identify a property that differentiates one from the other. Gather a collection of aluminum bolts and steel bolts, and make sure that both sets are identical. Then, mix the bolts thoroughly in a plastic bowl. Lower a bar magnet toward the bolts. The steel bolts are magnetic and will attract to the magnet as it gets close. As space on the magnet fills, remove the attracted bolts and place them in a separate container. Keep passing the magnet over the bowl until you have removed all of the steel bolts.
Skim Pickin's
Mix plastic marbles and glass marbles together in two large bowls. Take one bowl for yourself and give the other bowl to a partner. Tell your partner he or she will have to separate that mixture by hand and that you will separate the mixture using only a cup of water. Predict who will be able to separate the mixture faster. Ready a timer, and fill a large cup with water. Start the timer, and let your partner begin picking out the plastic marbles. Pour the cup of water into your bowl and watch as the plastic marbles immediately float to the surface while the glass marbles remain at the bottom.
Mystery Mixture
Have a friend create a batch of mystery mix for you to separate. Your friend can use any or all of the following ingredients: water, sand, sugar, soil and vegetable oil. When your friend presents the mixture, split it into several samples and perform experiments to identify its individual components. For example, if water is present, you can first try filtering the solution through a coffee filter to remove any sand or dirt. You could then boil the water to reveal the presence of sugar. If the mixture is dry but looks oily, you can add water to make the oil rise to the surface, and then skim it off.
- Science Web: Mixing and Separating
- Frostburg State University: Separating Mixtures
- Science World: Mysterious Mixtures
Cite This Article
Cascio, Christopher. "Fun Experiments For Separating Mixtures" sciencing.com , https://www.sciencing.com/fun-experiments-separating-mixtures-13769/. 9 March 2018.
Cascio, Christopher. (2018, March 9). Fun Experiments For Separating Mixtures. sciencing.com . Retrieved from https://www.sciencing.com/fun-experiments-separating-mixtures-13769/
Cascio, Christopher. Fun Experiments For Separating Mixtures last modified March 24, 2022. https://www.sciencing.com/fun-experiments-separating-mixtures-13769/
Recommended
Talk to our experts
1800-120-456-456
Separation of Mixture

Chemistry Experiment - Separation of Mixture
Introduction.
One issue that arises regularly in chemistry is the separation of the mixture's constituent parts. Separation theory is based on the idea that elements in a mixture might have various physical and chemical characteristics. The constituents are unadulterated elements or compounds. Every sample of a pure substance has the same qualities under the same pressure and temperature conditions. Every sample exhibits the same melting point, boiling point, solubility in a specific solvent, etc. When two or more non-reacting chemicals are combined, the mixture's constituent parts preserve their own identities and attributes, and these properties are generally exploited for the separation of the mixture’s constituent parts.
Table of content
Separation of Camphor
Separation of Sand
Separation of Common Salt
To separate the components of a mixture of sand, common salt, and camphor by sublimation.
Apparatus Required
Sand, common Salt (NaCl)
Camphor,
Filter Paper
Tripod Stand
Cotton Plug
Sublimation is defined as the conversion of a substance from the solid to the gaseous state without its becoming liquid
Camphor turns into vapour when heated. It is known as sublimation. Sand and regular salt, in comparison, do not sublime. So, by using the sublimation technique, camphor may be easily extracted from a combination that also contains ordinary salt and sand. Camphor vaporizes when heated, leaving the non-volatile components behind. Camphor turns from a liquid to a solid when it is cooled.
Solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent.
Insolubility is the opposite property, the inability of the solute to form such a solution.
Common salt is soluble in water, but sand is not soluble in water.
Sand particles stay on the filter paper when a mixture of common salt and sand in water is passed through the filter paper.
Evaporation is a technique used to separate out homogeneous mixtures.
Common salt can be extracted by evaporation from a solution of common salt in water.
Sublimation, filtration, and evaporation procedures are used to separate the various components of a mixture of camphor common salt and sand.
Procedure
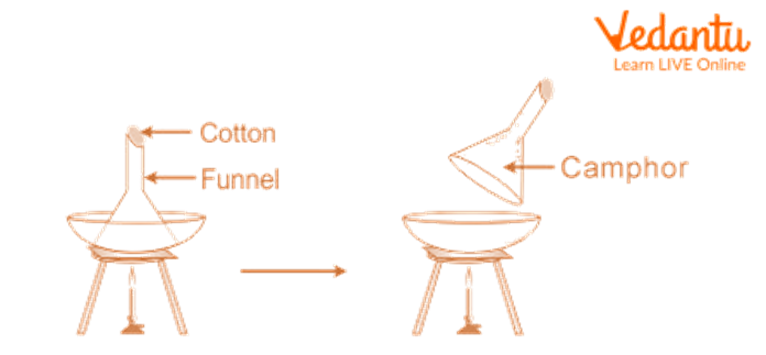
1. separation of camphor.
Place the sand, Camphor, and common salt combination in a China dish, then top it with an upside-down funnel.
Cover the funnel's opening with cotton.
Gently heat the China dish by setting it over a tripod stand.
Track the adjustments. Camphor is seen to go through sublimation, meaning that it produces vapours that condense on the funnel's cooler sides.
Remove the condensed camphor from the funnel's interior walls with a scraper.
Sand and regular salt are present in the mixture that does not sublimate and remains in the China dish.
1. Separation of Sand
Now, we will add water to the non-sublimate mixture.
Using a clean glass rod, stir the mixture.
Filter the mixture.
Sand is collected as the residue on the filter paper, whereas a common salt solution is obtained as the filtrate.
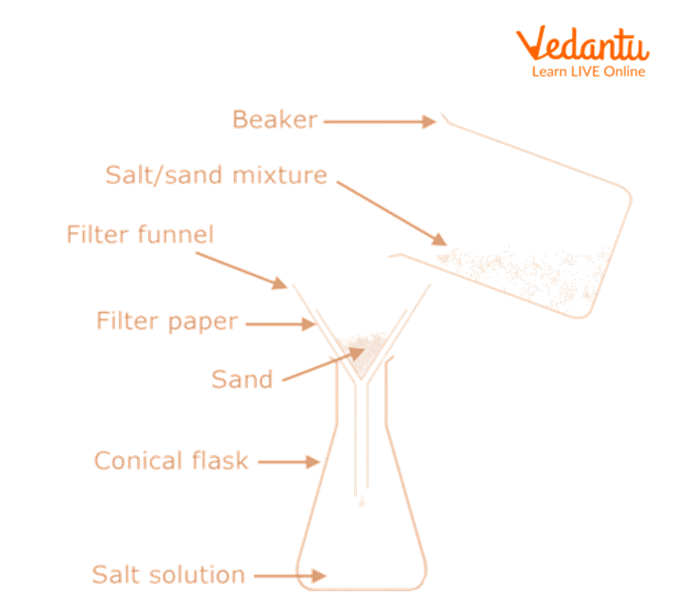
3. Separation of Common Salt
Take the filtrate and put it in a China dish.
Dry the filtrate to the point where sodium chloride (common salt), which is left behind as the water evaporates, is left in the China dish.
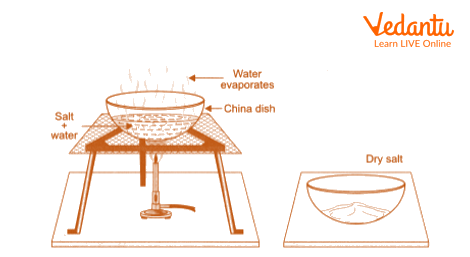
Separation of Salt
Observations
Sublimation, Filtration, and Evaporation are used to separate the mixture of Camphor, sand, and table salt.
Precautions
Be careful during heating experiments.
Keep the flame low if you are using a Bunsen burner.
Be careful when heating camphor, as it is volatile.
Add a cotton plug to the funnel's end to prevent the escape of camphor vapours.
Take care while filtering so that the filter paper does not tear off.
Lab Manual Questions
1. In the experiment mentioned above, you saw the transformation of solids into vapours. Do you think this shift is physical or chemical?
Ans. Directly converting solids to vapours constitutes a physical change.
2. Can any method be used to separate a mixture's two water-soluble components? Justify your response.
Ans. It is possible to separate two components of a combination based on their different levels of solubility in water if they are soluble in water. The crystallisation will be the method used. Chromatography is an additional method that may be employed.
3. What factors, in your opinion, could cause some solids to convert directly to vapours or vice versa?
Ans. Some solids convert directly to vapours or vice versa. This is because of the solid's high vapour pressure in the liquid form. There is essentially no liquid state.
4. What would the separation process have been like if the mixture had been dissolved in water in the initial step?
Ans. Since both Camphor and sodium chloride are water-soluble, if the combination had been dissolved in water in the first stage, their separation would not have been possible.
Viva Questions
1. What do you understand about separation Techniques?
Ans. A mixture is made up of two or more different forms of matter that may be present in different proportions and that can be physically separated using techniques that separate the mixture's components based on their physical characteristics.
2. Why does the mixture need to be separated?
Ans. The mixture needs to be separated because
To remove the unnecessary components from a combination, various components of the mixture are separated.
To separate more than one useful component from a mixture.
To acquire pure materials.
For instance, grain bought in stores may come with a variety of contaminants, including bits of stone, husk, broken grains, etc.
3. What are the differences between Pure substance and mixture?
Ans. The differences between Pure substance and Mixture are as follows:
4. What type of mixtures are separated by the technique of crystallisation?
Ans. The types of mixtures that are separated by the technique of crystallisation are:
This method is used to separate a solid-liquid mixture.
When a solid is dissolved in a liquid, it can be separated from the mixture by evaporating the mixture that leaves behind crystals of the solid.
For instance, crystals of alum (phitkari) are separated from impure samples, and sea salt obtained from the sea is removed from impurities.
5. What do you mean by sublimation?
Ans. A substance can sublimate when it moves straight from the solid to the gas phase without first going through the liquid phase.
6. How will you separate sand and water from their mixture?
Ans . Filtering Technique: A filter paper is used to filter a sand and water mixture (a filter with very fine pores). The filter paper traps larger sand particles, separating them from the water.
7. Give two examples other than camphor, which undergoes sublimation.
Ans. Ammonium Chloride and Iodine are the two examples which undergo sublimation.
8. Is it possible to separate the mixture of Camphor and Naphthalene by sublimation?
Ans. No, it is not possible to separate the mixture of Camphor and Naphthalene by sublimation because both are sublimely solids, and they sublime on heating.
9. How to separate cream from milk?
Ans. By centrifugation technique, we can separate cream from milk.
10. What are the techniques used to separate the components of the mixture?
Ans. The techniques used to separate the components of the mixture are:
Evaporation
Distillation
Filtration or Sedimentation
Separating Funnel
Magnetic Separation
Practical Questions
A sample of a mixture that is heterogeneous is:
Oil in water
Sugar solution
Salt in water
Ans. Oil in water is a heterogeneous mixture.
The following technique can be used to separate the salt and sand aqueous mixture.
Centrifugation
Sublimation
Ans. Filtration is the technique that can be used to separate the salt and sand aqueous mixture
When water is dissolved with sodium chloride and ammonium chloride, and then filtered, the residue is.
Sodium Chloride
Ammonium Chloride
Ans. When water is dissolved with sodium chloride and ammonium chloride, and then filtered, the residue is none.
How could salt, iron filings, and sulphur be separated from one another?
Magnet, dissolve in water, filter, crystallise.
Magnet, filter, decant, crystallise.
Dissolve in water, filter, crystallise, and decant.
Decant, crystallise, filter, dissolve in water
Ans. The magnet will separate the iron fillings from the mixture of salt and sulphur. Then we will dissolve the mixture in water and filter out the mixture using filtration technique. As a residue, we will get sulphur and salt solution as a filtrate. Follow up the crystallisation process and we will get the crystals of common salt.
Brine solution is
milk in water
an aqueous solution of potassium hydroxide
a concentrated solution of sodium chloride
molten butter
Ans. Brine is a concentrated solution of sodium chloride
Name the material that sublimates and is used to protect warm clothes.
Neem Leaves
Naphthalene
Ans. Naphthalene is the material that sublimates and is used to protect warm clothes.
Which one of the following is a pure compound?
Ans. Sugar is a pure compound
Common salt and water can be entirely separated using the procedure of
Ans. Common salt and water can be entirely separated using the procedure of evaporation.
The components of the compound can be separated by using
Physical method
Chemical method
Cannot be separated by using any method
None of the above
Ans. The components of the compound can be separated by using the chemical method
An example of a homogeneous mixture is
Sugar Solution
Sand in water
Ans. Sugar Solution is an example of a homogeneous mixture
Separation techniques are those techniques that can be used to separate two different states of matter such as liquids and solids. Separation processes or a separation method or simply a separation is methodology to attain any mass transfer phenomenon that converts a mixture of substances into two or more distinct product mixtures. Separation is an important asset to purify components of interest from mixtures.
FAQs on Separation of Mixture
1. What are the differences between Homogeneous and Heterogeneous mixtures?
The differences between Homogeneous and Heterogeneous mixtures
2. What is the difference between Evaporation and Crystallisation?
The difference between Evaporation and Crystallisation is as follows
3. What do you understand by Vaporization?
Vaporisation can be defined as the process in which the liquid state changes into the vapour state.
4. What are the characteristics of mixtures?
The characteristics of mixtures are: -
The mixture's composition is not fixed.
Energy is neither created nor evolved to create a mixture.
The melting and boiling points of the mixture are not fixed.
The qualities of the components are retained in the mixture.
Simple physical techniques can be used to separate the mixture's components.

FREE K-12 standards-aligned STEM
curriculum for educators everywhere!
Find more at TeachEngineering.org .
- TeachEngineering
- Element, Mixture, Compound
Hands-on Activity Element, Mixture, Compound
Grade Level: 11 (10-12)
Time Required: 30 minutes
Expendable Cost/Group: US $2.00
Group Size: 4
Activity Dependency: Separating Mixtures
Subject Areas: Chemistry
Activities Associated with this Lesson Units serve as guides to a particular content or subject area. Nested under units are lessons (in purple) and hands-on activities (in blue). Note that not all lessons and activities will exist under a unit, and instead may exist as "standalone" curriculum.
Te newsletter, engineering connection, learning objectives, materials list, worksheets and attachments, more curriculum like this, introduction/motivation, vocabulary/definitions, investigating questions, user comments & tips.

Materials scientists and mechanical engineers focus on understanding the nature and properties of varying materials so that they can iterate upon them and create more robust products. They take advantage of the varying strengths and abilities of different materials to make composites with significantly different physical or chemical properties. Understanding the properties of a given material, element, component, or composite is a critical part of the engineering design process.
After this activity, students should be able to:
- Distinguish and describe the three types of matter: elements, compounds, mixtures.
- Define pure and impure materials.
- Give some examples of elements, mixtures, and compounds.
- Explain the different properties of each group of materials.
- Explain how chemical engineers use these terms when solving problems related to water purification and distillation of crude oil.
- Explain how material and mechanical engineers use these terms regarding creating new composite materials.
- Explain what metal alloys are and explain the significance of metal alloys in material science and material engineering.
- Give some applications of non-metal alloys.
Educational Standards Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards. All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN) , a project of D2L (www.achievementstandards.org). In the ASN, standards are hierarchically structured: first by source; e.g. , by state; within source by type; e.g. , science or mathematics; within type by subtype, then by grade, etc .
International technology and engineering educators association - technology.
View aligned curriculum
Do you agree with this alignment? Thanks for your feedback!
State Standards
Texas - science.
To share with the entire class:
- 20 sets of bolts, nuts and washers
- 9 plastic dishes
- tape and marker, to number dishes
- four examples of elements:
- aluminum (one small sheet of foil)
- copper (a small piece of wire or tubing)
- iron (filings or a magnet)
- carbon (in the form of a pure carbon pencil or graphite)
- four examples of compounds:
- water, or H 2 0 (~100 mL)
- table salt, or NaCl (~10g)
- baking soda, or NaHCO 3 (~10g)
- eggshell or a seashell, or CaCO 3
- four examples of mixture, both homogeonous and heteogenous:
- inflated Ziploc bag (as an example of air)
- bottle of Coke or other soda
- salad dressing (such as a vinegarette made of oil and water)
- Data Table , one per student
We are all completely surrounded by matter. To better understand this matter—how it affects you, how you affect it and how it can be manipulated to our benefit—we need get a basic understanding of the types and properties of matter. The diversity of the matter in the world and in the universe is astounding. If we are to understand this diversity, we must start with a way of organizing and describing matter.
All matter is made of elements that are fundamental substances that cannot be broken down by chemical means. An element is a substance that can not be further reduced as to simpler substances by ordinary processes. In essence, an element is a substance consisting of one type of atom.
A compound is a pure substance composed of two or more different atoms chemically bonded to one another. That means that it can not be separated into its constituents by mechanical or physical means and only can be destroyed by chemical means.
A mixture is a material containing two or more elements or compounds that are in close contact and are mixed in any proportion. For example, air, sea water, crude oil, etc. The constituents of a mixture can be separated by physical means like filtration, evaporation, sublimation and magnetic separation. The constituents of a mixture retain their original set of properties. Further, mixtures can be classified to homogeneous and heterogeneous mixtures. A homogeneous mixture has the same uniform appearance and composition throughout its mass. For example, sugar or salt dissolved in water, alcohol in water, etc. A heterogeneous mixture consists of visibly different substances or phases. The three phases or states of matter are gas, liquid and solid. A heterogeneous mixture does not have a uniform composition throughout its mass.
New materials are among the greatest achievements of every age and they have been central to the growth, prosperity, security and quality of life of humans since the beginning of history. New materials open the door to new technologies, whether in civil, chemical, construction, nuclear, aeronautical, agricultural, mechanical, biomedical or electrical engineering.
The study of metal alloys, which are mixture of different metals, is a significant part of materials science and material engineering. Of all the metallic alloys in use today, the alloys of iron (steel, stainless steel, cast iron, tool steel, alloy steels) make up the largest proportion both by quantity and commercial value. Iron alloyed with various proportions of carbon gives low, mid and high carbon steels. For the steels, the hardness and tensile strength of the steel is directly related to the amount of carbon present, with increasing carbon levels also leading to lower ductility and toughness. The addition of silicon and graphitization produce cast iron. The addition of chromium, nickel and molybdenum to carbon steels (more than 10%) gives us stainless steels.
Other significant metallic alloys are those of aluminium, titanium, copper and magnesium. Copper alloys have been known for a long time (since the Bronze Age), while the alloys of the other three metals have been relatively recently developed. The alloys of aluminium, titanium and magnesium are also known and valued for their high strength-to-weight ratios and, in the case of magnesium, their ability to provide electromagnetic shielding. These materials are ideal for situations in which high strength-to-weight ratios are more important than bulk cost, such as in the aerospace industry and certain automotive engineering applications.
Other than metals, polymers and ceramics are also an important part of materials science. Polymers are the raw materials (the resins) used to make what we commonly call plastics. Plastics are really the final product, created after one or more polymers or additives have been added to a resin during processing, which is then shaped into a final form.
Another industry application is the making of composite materials. Composite materials are structured materials composed of two or more macroscopic phases. Applications range from structural elements such as steel-reinforced concrete, to the thermally insulative tiles that play a key and integral role in NASA's Space Shuttle thermal protection system, which protects the surface of the shuttle from the heat of re-entry into the Earth's atmosphere. One example is reinforced carbon-carbon (RCC), The light gray material withstands reentry temperatures up to 1510 °C (2750 °F) and protects the Space Shuttle's wing leading edges and nose cap. RCC is a laminated composite material made from graphite rayon cloth and impregnated with a phenolic resin.
Other examples can be seen in the "plastic" casings of television sets, cell phones and other modern devices. These plastic casings are usually a composite material.
Before the Activity
Gather materials and make copies of the Data Table , one per student.
Prepare nine plastic dishes of bolts, nuts and washers as described below. Place them on a table (called Table A). Indicate the dish numbers in some way, such as with tape and a marker.
- Dish 1: 4 washers
- Dish 2: 4 bolts
- Dish 3: 4 nuts
- Dish 4: combine 1 nut with 1 bolt (4 sets)
- Dish 5: combine 2 nuts with 1 bolt (4 sets)
- Dish 6: combine 1 nut and 1 washer with 1 bolt (4 sets)
- Dish 7: 1 washer, 1 nut, 1 compound as in dish 5 and 1 compound as in dish 6
- Dish 8: 2 washers, 1 nut, and 2 bolts
- Dish 9: 1 compound as in dish 4 and 2 compound as in dish 5.
Place the examples of elements, compounds, and mixtures on another table (called Table B) and label them. You may also label the compounds with their specific chemical formula (for example, table salt would be NaCl).
With the Students
- Divide the class into groups of four students each. Hand out the blank data tables.
- Direct each student's attention to Table A. The sets of washers, bolts and nuts can be used to convey the concept of elements, mixtures and compounds. Explain to the students that if they think each washer, bolt and nut as an individual atom then the contents of dishes 1, 2 and 3 are elements because they are all the same atom and that can not be further reduced as to simpler substances. The contents of dishes 4, 5 and 6 are compound because they show one substance composed of two or more different atoms chemically bonded to one another and the contents of dishes 7, 8 and 9 are mixtures because they are materials containing two or more elements or compounds and are mixed in any proportion.
- After the discussion about Table A, direct each group to go through Table B and compare and contrast the different items on the table and make a list of their discussion. Have them categorize the materials in each dish as element, homogenous mixture, heterogeneous mixture or compound, recording this in their data tables. Ask students to discuss their lists. (Expect some to categorize the materials as elements, mixtures and compounds.)
- At this point, explain the different types of matter, using the classroom board as needed. Then, discuss all the materials on Table B again and separate them to classes of elements, mixtures (homogeneous and heterogeneous) and compounds.
compound: A pure chemical substance consisting of two or more different chemical elements.
element: A substance consisting one type of atom.
heterogeneous mixture: A mixture that consists of visibly different substances or phases.
homogeneous mixture: A mixture that has the same uniform appearance and composition throughout its mass.
mixture: A substance consisting of two or more materials that aren't chemically combined.
solution: A homogeneous mixture composed of two or more substances.
Questions : Ask students the Investigating Questions as part of a concluding class discussion. Students' answers and contributions to the discussion reveal their comprehension of the activity concepts. Alternatively, ask students to individually answer the questions in the form of a final written test.
- Describe the three types of matter: elements, compounds and mixtures.
- Give some applications of nonmetal alloys.

Students learn how to classify materials as mixtures, elements or compounds and identify the properties of each type. The concept of separation of mixtures is also introduced since nearly every element or compound is found naturally in an impure state such as a mixture of two or more substances, and...

To gain an understanding of mixtures and the concept of separation of mixtures, students use strong magnets to find the element of iron in iron-fortified breakfast cereal flakes. Through this activity, they see how the iron component of this heterogeneous mixture (cereal) retains its properties and ...

Contributors
Supporting program, acknowledgements.
This digital library content was developed by the University of Houston's College of Engineering under National Science Foundation GK-12 grant number DGE 0840889. However, these contents do not necessarily represent the policies of the NSF and you should not assume endorsement by the federal government.
Last modified: January 11, 2019

IMAGES
VIDEO
COMMENTS
A heterogeneous mixture is one in which the composition of the mixture is not uniform all the way through. Suspensions, colloids, and emulsions are types of heterogeneous mixtures. When investigating these types of mixtures, students learn how to differentiate between a suspension and a colloid and what makes a colloid different from an ...
What is a Mixture? A mixture is a substance in which two or more substances are mixed but not chemically joined together, meaning that a chemical reaction has not taken place.. Mixtures can be easily separated and the substances in the mixture keep their original properties. Imagine mixing skittles and full size marshmallows, the individual components (skittles and marshmallows) could easily ...
Plus there's a bonus in here, you may not have even realized was a separation of mixtures lab! To preface, I teach 6 methods for separation of mixtures: distillation, evaporation, chromatography, precipitation reaction, separatory funnel and filtration. Chromatography Lab. For this separation of mixtures lab, you'll only need a few materials.
processes used to separate and analyze complex mixtures based on differences in their structure or composition. During chromatography, a mixture is moved over a stationary material, called the stationary phase. The mixture that flows over the material is called the mobile phase. The different parts that make up the mobile
Chances are that you separate mixtures often. For example, any time you separate laundry or pick a topping off a pizza or drain a batch of freshly cooked pasta, you are separating a mixture. A mixture is a combination of substances that do not react chemically when they are mixed. According to this definition, a solution -- such as sugar water -- is a mixture just the same as a mixture of ...
Dive into a chemistry experiment focused on the separation of mixtures. Discover and apply various techniques to effectively separate different components within a mixture, gaining practical insights into fundamental principles of chemical separation. ... Separation theory is based on the idea that elements in a mixture might have various ...
Learn about mixtures and solutions with this fun, hands-on science challenge! Explore creative ways to separate different mixtures like rocks and gravel, mud and water, and the tricky sand and salt combo ... Before you get started, list as many different ways as you can think of to separate the mixture. The point of this experiment, is to try ...
Further, mixtures can be classified to homogeneous and heterogeneous mixtures. A homogeneous mixture has the same uniform appearance and composition throughout its mass. For example, sugar or salt dissolved in water, alcohol in water, etc. A heterogeneous mixture consists of visibly different substances or phases.
Separating mixtures is a lesson with fun science experiments. Kids will learn how to separate mixtures, sieving, filtering, evaporating etc. ... filtration and evaporation. Try these fun science experiments carefully with the help of an adult. Separating Mixtures . Depending on the type of mixture the individual substances in a mixture can be ...
It is a fascinating look at what particles make up the heterogeneous mixture of sand and in what percentages. Heterogeneous Mixture Experiment Supplies. All you need for this experiment is a hand lens (magnifying glass), a toothpick, and sand. Experiment Procedure. Spread approximately 1 teaspoon of sand out on a white piece of paper in a ...