School Finder


10 Accredited Online Colleges in Clinical Research
- Phone Vs. Text Vs. Email
- The Everlasting Lessons of Mr. Rogers
- Fake Schools, Fake Degrees: Avoiding Diploma Mills
- Silver Surfers: Internet Usage Among Older Generations
- Understanding Accreditation
- How To Get A Free Education
Accredited Online Program Rankings
- Accredited Online Colleges That Offer Free Laptops
- Top Accredited Online Colleges That Have Low Tuition
- Cheapest & Easiest Masters Degrees Online
- Cheapest Accredited Online Colleges for Diagnostic Medical Sonography
- Top Accredited Online Colleges for Early Childhood Education
- Top 30 Easiest Online PhD Programs
- Top Accredited Online Colleges for Physical Therapy
- Top Free Accredited Online Courses With Certificates
- Easiest Online College Programs for Undergrads
…View more
Accreditation Resources
- Accreditation List
- List of American Psychological Association (APA) Accredited Programs in Alabama
- Accredited Online Bible College Programs
- The Different Online Learning Modes For Online Degree Programs
Accredited Online Degrees
- The Importance of Online Accreditation
- The Difference Between Regional and National Accreditation
- The Advantages to Accredited Online Degree Programs
- An Overview of Accredited Online Associate Degree Programs
- An Overview of Accredited Online Bachelor Degree Programs
- An Overview of Accredited Online Master Degree Programs
- An Overview of Accredited Online Doctoral Degree Programs
- Engineering Degree Basics
Choose one of our top 10 accredited online colleges in clinical research for a career that is both personally and financially rewarding.

An online degree in clinical research allows you to gain the knowledge necessary to develop and conduct clinical research trials. Moreover, the knowledge and skills you gain enable you to work in various fields, from research ethics to research project management to regulating medical products.
In fact, clinical researchers are integral members of teams developing new medicines, devices, and services that save thousands of lives each year. With your contributions, you can make the world a healthier and safer place while improving the quality of life of untold patients around the world.
Featured Programs
See Also: 9 Tips For Balancing Work, Life, and Academics: Navigating the Challenges of Pursuing an Online Master’s Degree
What Will You Learn With a Degree in Clinical Research?
An online clinical research masters degree heavily focuses on the how’s and why’s of research. The programs listed below teach you the various steps in conducting a clinical research study. Just as important, you’ll learn about the ethics of conducting research.
While some people dismiss the importance of courses in ethics, medical research is full of examples of studies that caused physical, emotional, or psychological harm to research participants. Ethics courses ensure you understand the risks and potential harm that could result from clinical research.
Other topics you might study include:
- Regulatory management issues
- Regulatory development
- Clinical research methods and design
- Clinical research data management
- Clinical trial budgets and contracts
Most of the clinical research online programs on this list lead to master’s degrees, but there are a few certificate programs as well. While each program may offer different courses, many of the programs share a common core. Examples of specific courses you’re likely to see while enrolled in one of our top 10 accredited online colleges in clinical research include:
- Epidemiology and Biostatistics
- Research Methods
- Introduction to Drug Development
- Regulatory Affairs in Drug Development
- Regulatory Writing and Electronic Submissions
Keep in mind that some of these research degrees online allow you to choose a specialization in clinical research. With these programs, you’ll take additional classes to gain expertise in the specialization area. You will also be able to explore areas of interest through elective courses and clinical research with human subjects.
It should be noted that some online clinical research programs might have in-person requirements. You may have to participate in intensive on-campus coursework once or twice a semester. You might also need to complete practicum or internship experiences in which you gain real-world training while studying under the direction of an experienced clinical researcher.
See also: Top Nationally Accredited Online Colleges For Nursing
What are the Typical Admission Requirements for a Clinical Research Program?
The admissions requirements for these programs vary somewhat from one school to the next. For example, some schools might require that you have a bachelor’s degree in a research field. Others might require that you have a bachelor’s degree in any field.
Additionally, some schools might have more stringent academic requirements. For example, one university might require applicants to have a 3.25 undergraduate GPA, while other programs might only require a 3.75 GPA. Some programs may require you to take specific undergraduate classes, like advanced statistics or research methods courses. You may need to provide evidence of working on a relevant research project as well.
Despite these and other differences, there are some common graduate program admission requirements for enrollment in clinical research. These might include:
- Submitting official transcripts from all colleges and universities you’ve attended
- Providing a personal statement or a statement of career goals
- Providing letters of recommendation from academic and/or professional references
- Completing an interview with the academic program director
Additionally, you may have to complete a readiness assessment that gauges your understanding of critical clinical research competencies.
See also: Accredited Online Colleges in Public Health
What Are the Best 10 Accredited Online Colleges in Clinical Research?
Accredited Online College uses the most recent data from the Department of Education’s National Center for Education Statistics to develop the rankings you see below. Each program is scored individually. It’s then compared to all other universities offering that degree to determine the final score you see by each ranking.
Accredited Online College considers several factors for these rankings. These include the program’s affordability, the student-to-faculty ratio, and the number of on-campus and online programs. For more information, see our methodology page.
Let’s get to our list of the clinical research degree online options awaiting you!
See Also: Financial Aid Tips for Online College
1. The Ohio State University

At number one on our list of clinical research degree programs is the Master of Clinical Research from The Ohio State University.
This program is available through the school’s College of Nursing and is offered entirely online. This program stands out from the pack because the coursework is multidisciplinary and is taught by instructors in both the College of Nursing and the College of Pharmacy. This interdisciplinary approach gives you a broader base of understanding of clinical research.
Some of the courses you’ll take include:
- Fundamentals of Medical Product Development and Regulation Affairs
- Responsible Conduct of Research
- Clinical Research Study and Site Management
- Clinical Research Design and Methods
Many courses in this program are asynchronous, so you can attend to your studies when it’s convenient for you.
You can specialize your coursework by focusing on either the Clinical Research Management Track or the Regulatory Affairs track.
The Clinical Research Management Track focuses on teaching you the processes involved in conducting and managing clinical research. Upon completing this track, you will be ready for employment as a clinical research coordinator or clinical research associate.
Alternatively, the Regulatory Affairs Track is geared toward online students who want to focus on the regulation of research. You’ll also focus on the compliance and communication aspects of clinical research.
See Also: Accredited Pharmacy Schools Online
2. University of North Carolina Wilmington

If you’re searching for a career in clinical research but don’t want to spend your days in the laboratory, then the Master of Science in Clinical Research & Product Development from the University of North Carolina Wilmington may be the perfect degree for you.
This program was designed for people who want to work in management and oversight positions in clinical research. Although you won’t be required to participate in extensive laboratory experiences to graduate, you will be taught the research process and stages involved by examining a study from start to finish.
Twelve courses, which include the capstone course necessary for graduation, compose this master of clinical research online . Classes in this program include:
- Clinical Research Monitoring and Ethics
- Advanced Scientific Writing and Interpreting Medical Literature
- Clinical Research Trial Design and Data Management
- Regulatory Affairs and Quality Management
Many other courses are required, including those focusing on pharmacology, ethics, and current issues in regulatory development and management.
UNC’s admission team is looking for applicants with a BA or BS in life science, health care discipline, or mathematics/statistics. You must also submit supporting documents with your application, including a resume or curriculum vitae, a personal essay, and three letters of recommendation.
3. Arizona State University-Tempe

The Online Master of Science in Clinical Research Management from Arizona State University focuses on providing a top-notch education. You get that education with built-in flexibility to accommodate your busy work and home life.
This master’s degree program includes 11 courses equaling 33 credit hours of study. Each class lasts 7.5 weeks. This accelerated format positions you to take fewer classes at a time yet still complete your degree on a reasonable timetable. In fact, you might be able to finish this master’s program in just one year.
You’ll take many different courses in this program, including:
- Clinical Research Data Management and Technology Implementation
- Fundamentals of Regulatory Affairs
- Scientific and Research Review Boards
- Responsible Conduct of Clinical Research
- Drug Discovery, Development, and Regulations
Furthermore, you must complete a capstone project during the final term of the program. The project is a cumulative learning experience that combines the knowledge and skills you’ve learned throughout the program to address a crucial issue in clinical research. In some cases, your capstone project might be published in relevant journals and other publications.
Competitive applicants must already have an undergraduate or graduate degree. Furthermore, anatomy, physiology, and medical terminology coursework are highly recommended
ASU provides opportunities for you to connect with future employers. This might make it easier to land your first job after graduation.
4. St. Cloud State University
Success in a career demands more than just the technical knowledge required to prove competence. Your success also requires the leadership and communication skills that distinguish a mediocre hire from a company superstar.

St. Cloud State University appreciates this distinction, which is why it created the Master of Science in Applied Clinical Research. As a student in this program, most of your education will be on the technical aspects of clinical research. However, the school also prepares you to thrive in leadership positions in clinical research.
This online clinical research degree is the only clinical research program on this list geared specifically toward the use of medical devices. With specialization in specific areas of clinical research comes the opportunity to land your ideal high-paying job. You’ll explore topics like:
- The history of protections and regulations
- The logistical and scientific requirements of clinical research
- Ethical practices in clinical research
- Site management of clinical research
- Designing medical device clinical trials
This program features two start dates each year: one in the spring and one in the fall. As a student of this university, you’ll be able to select either a full- or part-time route to complete your degree.
5. Eastern Michigan University

Eastern Michigan University offers two ways in which you can gain expertise in clinical research: one is through a Graduate Certificate in Good Clinical Practices, and the other is through a Master of Science in Clinical Research Administration.
The graduate certificate consists of 15 credit hours of coursework. If you opt for this track, you’ll take classes such as:
- Clinical Study Administration I & II
- Advanced Topics in Clinical Study Management
The Master of Science in Clinical Research Administration program comprises 34-35 credit hours of coursework, with 19 credit hours making up the core. The required coursework includes many of the same classes as the graduate certificate, along with:
- Ethical Issues in Clinical Research
- Regulatory Affairs in Drug Development I
- Graduate Seminar in Drug Development
This degree allows you to choose between a thesis and a research project option. The thesis option is six credit hours and takes place during your final year in the program. The research project is worth four credits and also takes place during the final year.
6. University of Texas Rio Grande Valley

The Master of Science in Health Sciences from the University of Texas Rio Grande Valley has a concentration in Clinical Laboratory Sciences. This online program was created specifically with certified medical laboratory scientists and medical technologists in mind. Most students need only around one year of full-time study to complete the program. It’s a fast-track way to advance your career.
This MS degree includes 36 credit hours, with courses lasting around seven weeks. Classes you’re likely to take in this clinical research program online include:
- Research in Health Sciences
- Health Computer Information Systems
- Health Care Policy, Organization, and Financing
- Introduction to the Health Care System and Quality Improvement
- Professional Proposal Writing
What’s more, the tuition is reasonably priced at $444 per credit hour, which means that the degree, in total, is expected to cost students just under $16,000.
You must already have a bachelor’s degree from a regionally-accredited institution to apply. You must also have a 3.0 GPA or higher for your last 60 credits of study.
7. Rutgers University

The Department of Health Informatics at Rutgers University offers a Master of Science in Clinical Research Management with hybrid learning opportunities. Some courses are offered online, while others may require attendance in person.
This program offers three concentration areas. These include:
- Clinical Research Management
- Drug Safety and Pharmacovigilance
- Academic Clinical Research Management
In any case, this is a 36-credit-hour, multi-disciplinary program. Depending on your chosen concentration area, you will need anywhere from 16 months to three years to complete your studies. During that time, you will explore topics like:
- Scientific Concepts and Research Design
- Clinical Trial Operations
- Study and Site Management
- Communication and Teamwork
- Ethical and Participant Safety Considerations
Each course included activities that challenge you and engage you in the content. With experiential learning activities and expert professors, you’ll have many opportunities to expand your understanding of critical issues in clinical research.
Moreover, this program follows the cohort model, so you’ll move from one class to the next with the same group of classmates. This model facilitates a creative and supportive learning environment while bolstering your connections with peers.
8. Drexel University

Drexel University’s Master of Science in Clinical Research is specifically designed for health professionals like fellows, residents, and clinicians. Over the course of the program, you’ll take an interdisciplinary course load taught by attorneys, researchers, administrators, and other professionals. Though most students need two to three years to complete the program, you can take as many as seven years to finish.
You’ll take 12 courses for a total of 36 credits. These courses may include:
- Epidemiology
- Fundamentals of Compliance
- Innovative Product Development
- Compliance and Monitoring Issues
- The History of Misconduct in Biomedical Research
Unlike many programs in this field, this one does not require a thesis or internship. And since it’s an online program, there are no residency requirements to complete on-campus coursework.
However, you must complete a real-world clinical experience. This requirement involves mentored research with a doctoral-level advisor. Their guidance will assist you in developing the necessary skills to guide and conduct clinical research of your own.
9. George Washington University

The Bachelor of Science in Health Sciences degree from George Washington University is a 120-credit program that’s available entirely online. As part of this clinical research associate degree online , you’ll take 17 core courses worth 51 credits. You must also take nine credits of electives in clinical research for a total of 60 credits needed to complete the major area studies.
Below is a sample of courses you will take:
- The Business of Clinical Research
- Pathophysiology
- Processes of Clinical Research
- Introduction to Statistics for Health Science
- Introduction to Regulatory Affairs
Since this is a fully online program, you should be self-directed and have the self-discipline required to stay on top of your studies. Likewise, you should be interested specifically in working in the clinical trials industry.
You must have a minimum 2.5 GPA for your undergraduate studies to apply. You must also submit a resume or curriculum vitae, a personal statement detailing why you want to pursue this degree, and at least one letter of recommendation.
10. Nova Southeastern University

The last entry in our ranking of the best online clinical research programs is from Nova Southeastern University.
If you’re a healthcare professional already involved in clinical research and want to gain the knowledge and expertise that a master’s degree can provide, then this Master of Health Science with a Clinical Research Concentration is the program for you. To graduate with this master’s degree, you are required to complete 39 credit hours of coursework.
The core curriculum of this program consists of 12 credit hours of classes like:
- Current Trends and Cultural Issues in Health Care
- Writing for Allied Health Professionals
The Clinical Research Concentration requires you to take nine specialty and practical courses for a total of 27 credit hours of clinical-research-specific coursework. These courses include:
- Qualitative Research Methods
- Quantitative Research Methods
- Research Ethics
- Applied Statistics
Furthermore, you must complete a developmental research project. This project allows you to demonstrate your clinical research skills in oral and written forms. A large component of this project is thesis research, which you’ll primarily do during the program’s final year. You must successfully defend your thesis to your committee to graduate.
Frequently Asked Questions
What kind of job can i get after completing a degree in clinical research .
So, you’re wondering what kind of job you’ll be qualified to apply for once you’re finished with your clinical research degree? This is a great starting place when deciding if a clinical research degree is even right for you! Various opportunities are available if you decide to put your time and money into earning one of these degrees.
The most commonly traveled route for graduates of clinical research degree programs is to become a clinical research associate or clinical research coordinator. Clinical research associates are part of the research team that is involved in various aspects of conducting clinical research. This includes gathering, selecting, and educating research participants and filling out various paperwork for running one of these trials.
Clinical research coordinators perform some of the same jobs as clinical research associates. They are more responsible for coordinating the various steps of research studies, which often happen simultaneously. Clinical research coordinators may also monitor others involved in the study to ensure they comply with the rules, regulations, and guidelines.
Many clinical research coordinators and clinical research associates have a background in the healthcare field. For example, nursing is a common background for these positions, as are other healthcare professions. Healthcare provides a solid background for clinical research studies because they involve the health and well-being of people.
With that said, laboratory and study sites are not the only places where you can put the knowledge and skills you learn in your clinical research degree to work. After graduation, you might work in a management position at a pharmaceutical or medical device company. Individuals with knowledge of the clinical research process are ideally placed to make decisions for the future directions of these companies.
Do I Need a Master’s Degree to Be a Clinical Researcher?
Though a master’s degree won’t hurt, it isn’t totally necessary to pursue a career in this field, either. As discussed in the next section, your education level strongly influences how much money you make. Yet, a master’s degree or doctorate isn’t required for all clinical research positions.
In fact, most clinical research jobs require a bachelor’s degree – about 60% . Some jobs in this field, like entry-level positions, don’t even require that. You might find a starter position with an associate’s degree, a certificate, or even some combination of college courses and a certificate.
However, these entry-level jobs are just that – introductory positions where you can get experience as you complete the educational requirements for a more advanced degree. If you want to keep advancing your career, you must continue pursuing additional education and training.
What Kind of Salary Can I Expect After Earning a Clinical Research Degree?
The second question you may be asking yourself, and for some, this may be the first, is, “How much can I earn with a clinical research degree?” Simply put, if you complete an online clinical research program , you might be well compensated for the time and effort associated with earning your degree.
Clinical research associates and clinical research coordinators, most of whom have bachelor’s or master’s degrees and work for a clinical research organization, earn a jaw-dropping $137,900 per year. Yes, you read that correctly – these individuals earn, on average, around $66.30 per hour. Even more astounding is that these are median salaries, meaning many individuals earn significantly higher salaries.
Many factors can affect the salary you can expect to earn. This includes your level of experience, the company at which you’re employed, and the state in which you reside. Your level of education also matters.
For example, if you get an online PhD in clinical research , you can likely command more money due to a higher level of education (though a doctoral degree alone isn’t a guarantee of a higher income). As another example, clinical research associates that work for government agencies, like public health offices or the Federal Drug Administration (FDA), tend to make less than those in the private sector, like research organizations.
According to O*NET OnLine, there are currently nearly 79,000 individuals employed in these positions. Additionally, the profession is expected to grow at an average rate of 4%-7% within the next ten years. This means that as you progress through your program of study, there should be jobs available upon your graduation.
Alternatively, if you decide to use your newly acquired skills to work for pharmaceutical companies, you’ll likely earn nearly $95,000 per year. Like clinical research associates, research coordinators, and other clinical research professionals, various factors go into the earning potential of this job, like location and the medical products you sell.
There are currently an estimated 280,700 individuals working as technical and scientific product sales representatives, and the profession is expected to grow at an average rate of 4%-7% in the next decade.
If you decide to enter management and eventually work up to a chief executive position, you’ll also have a bright financial outlook. Chief executives go by a variety of titles, including:
- Chief Operating Officer (COO)
- Chief Nursing Officer (CNO)
- Chief Financial Officer (CFO)
- Chief Diversity Officer (CDO)
- Chief Executive Officer (CEO)
Almost every company features these positions, and people who fill these roles are ultimately responsible for the direction and management of the organization.
With substantial responsibility often comes substantial compensation. The median income for chief executives is just under $179,520 per year. It’s estimated that around 283,900 people hold these positions. However, these careers may be more challenging than the others listed above. For example, these jobs are expected to decrease by about 2% in the next ten years.
IIf you want to pursue higher education, check out the Top Easy Accredited Online Master’s Programs and the 30 Accredited Online Colleges For Nursing .
- About AstraZeneca
Life at AstraZeneca
- Our Locations
- BioPharmaceuticals
- BioPharmaceuticals R&D
- Enabling Functions
- Information Technology
- International
- Oncology R&D
- Early Talent
- Application Hints & Tips
Search jobs by keyword(s)
Search jobs by location
Keyword Search
City, State, or ZIP
Discover your career with us
Want to start your career at the cutting-edge of medical innovation? To unleash creative ideas that stand out in a global market leader? And work with some of the brightest minds in the industry who will push you to be your best? All while building the personal and professional skills you need to lead our exciting, dynamic evolution? Join us and impact millions of lives, together.
Early Talent programmes
Read about and apply to our programmes using the links below.
Business Apprenticeships UK
Locations: Cambridge and Macclesfield, UK
Our business teams support the research and development of life-changing medicines for our patients every step of the way and help turn our most ambitious goals into reality. Functions such as Finance, Human Resources, Project Management and Supply Chain play a part in supporting our people and our processes. So, whether you have a passion you want to pursue or are still figuring it out, there’s endless opportunity to experience new things and learn from different areas of the business to discover what truly inspires and motivates you.
Digital, Technology Solutions, and Data Apprenticeships UK
In the AstraZeneca Information Technology (IT) organisation, we are passionate about driving technological innovation, continuously improving our IT environment to ensure our internal teams can do their best work and successfully deliver our business goals. Here, you’ll work with world-renowned talent and leaders on real projects to gain hands-on experience whilst also working towards your qualification.
Operations Apprenticeships UK
Locations: Macclesfield and Speke UK
Global Operations at AstraZeneca facilitates innovation and connection between our science laboratories and our patients. We provide a vital platform for our business to play a key role in the development, manufacturing, testing and delivery of our medicines across the globe. Here, you’ll learn and earn in a supportive team, guided by expert managers, mentors and buddies who will help you develop the skills you seek, while pushing and encouraging you to get the most out of your time with us.
Scientific Apprenticeships UK
We are a scientifically led business and our purpose is all about pushing the boundaries of what science can do. Our science apprenticeships involve working in our laboratories and being part of our journey to provide life changing medicines to patients all over the world – transforming lives for the better.
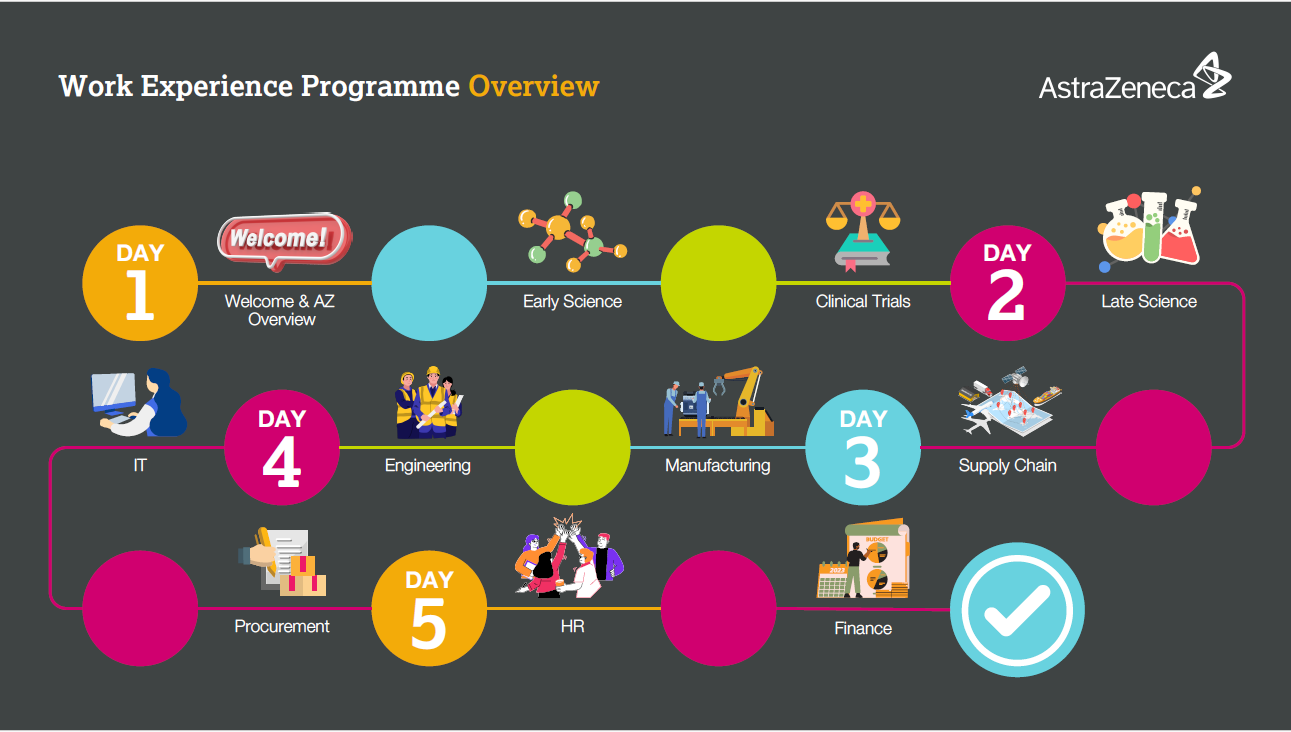
Year 10 work experience UK
Locations: Virtual Programme, UK
At AstraZeneca we recognise that access to work experience is important for students to gain valuable insights into different industries and to help them with their professional and personal development.
We are delighted that AstraZeneca will be hosting virtual work experience opportunities for Year 10 students in the UK during February, June and July 2025. Please see below for an overview of the week and you can register using this link - AstraZeneca work experience - sign up form
Year 12 work experience UK
Locations: Cambridge and Macclesfield – UK
At AstraZeneca we recognise that access to work experience is important for students to gain valuable insights into different industries and to help them with their professional and personal development
Engineering
Macclesfield Campus, Cheshire
Available opportunities will be advertised here January 2025
Pharmaceutical Technology & Development – Science
Research & Development – Science
Cambridge Biomedical Campus, Cambridge
Supply Chain
US Apprenticeships
- Canóvanas, Puerto Rico
- Coppell, Texas
- Gaithersburg, Maryland
AstraZeneca US produces innovative medicines that are used by millions of patients worldwide. Our purpose to better society is a source of motivation for our people, who share a passion for what science can do. Our 14 locations across the US make us an accessible company with incredible expertise in the medical field.
AstraZeneca will not discriminate against apprenticeship applicants or apprentices based on race, color, religion, national origin, sex (including pregnancy and gender identity), sexual orientation, genetic information, or because they are an individual with a disability or a person 40 years old or older. AstraZeneca will take affirmative action to provide equal opportunity in apprenticeship and will operate the apprenticeship program as required under Title 29 of the Code of Federal Regulations, part 30.
Internships (UK)
AstraZeneca is a place where we all help and empower each other. Our supervisors are committed to providing you with all the guidance and support you need to build your skills, confidence and capabilities. Our internships are for those who want to gain an insight into a particular sector or role within our global organisation. Varying in length and aim, our internships are the perfect opportunity to set yourself up for a successful career.
Leaders in Finance Tomorrow (US)
The Leaders in Finance Tomorrow (LIFT) is an AstraZeneca US internship program that provides the best and the brightest students with a unique opportunity to gain meaningful finance and accounting experience within our industry-leading business. As a young professional, you’ll be treated as such – given all the trust and empowerment you need to take on new challenges, but with all the help and guidance you need to succeed.
Pharmaceutical Technology & Development Undergraduate Industrial Placement Studentship (UK)
Our Pharmaceutical Technology & Development Undergraduate Industrial Placement Studentship (PT&D UIPS) introduces you to the world of ground-breaking drug development, placing you in highly dedicated teams committed to delivering impactful new medicines to patients. Here, you’ll gain an insight into what it’s like to work in a big biopharma, with exposure to people who truly care about your personal career development.
Research & Development Undergraduate Industrial Placement Studentship (UK & Sweden)
The Research & Development Undergraduate Industrial Placement Studentship (R&D UIPS) introduces undergrads to the world of drug discovery, embedding students within highly dedicated teams driven to create impactful new medicines. Working on projects with real patient implications, you could have the opportunity to contribute to making a meaningful, life-changing impact for millions of people around the world.
Student Workers & Internships (US)
Join AstraZeneca’s student workers or interns, and you’ll find a biopharmaceutical environment that’s full of unique opportunities and exciting challenges. Here, you’ll have the opportunity to pursue your areas of interest whilst equally developing a broad skillset and knowledge base to get the best out of your experience. You’ll be given meaningful projects from day one, and you’ll enjoy plenty of chances to network within your chosen business area, too.
Work Placement Student Programme (Sweden)
The Work Placement Student Programme provides the opportunity for you to undertake a 12-month placement in our Gothenburg site in Sweden that will introduce you to the world of innovative drug discovery pushing the boundaries of science to deliver life-changing medicines. You’ll get to do meaningful work in a pioneering research and development driven organisation.
Biometrics Graduate Programme
Locations: Canada, Poland, Spain, Sweden, UK, US
Collaborating cross-functionally with experts in a stimulating, fast-paced environment, you’ll have all the freedom, empowerment and support to add value in study design, data analytics and interpretation of our innovative portfolio of medicines. Applying your knowledge to accelerate your career and make an impact. Because here, you’re truly valued. Encouraged to speak up, ask questions, get stuck in and contribute in a meaningful way. Using your skills, passion and desire to learn, drive your development and achieve your personal and professional objectives.
Biopharmaceutical Development Graduate Programme
Locations: Cambridge, UK & Gaithersburg, US
Designed to help you transition from academia to industry, you’ll progress through different programme rotations – gaining valuable experience and insight across various functions within Biopharmaceutical Development to really push and expand your skills and knowledge. Working and collaborating on challenging technical projects across a range of therapeutic areas, you’ll find continuous opportunities to learn, develop and make a real impact. All whilst fuelling your curiosity, building lasting relationships, and working towards your personal and professional goals.
Data Science & Artificial Intelligence Graduate Programme
Locations: Cambridge, UK; Boston & Gaithersburg, US; Gothenburg, Sweden
We’re passionate about what the power of Data Science and AI can do. Right now, we’re on a journey to become a data-led enterprise that disrupts the industry and sets the standard. So, if you’re an innovative thinker with a passion for Data Science and AI, there’s never been a better time to be part of our Graduate Programme. Through the challenging, developmental placements, you’ll work closely with peers, mentors and experts to develop a broad set of skills You’ll work collaboratively, embracing new ideas and different perspectives. And you’ll bring creative, critical thinking to turn data and insights into actions that help develop life-changing medicines.
Local Graduate Programmes
In addition to our Global Graduate Programmes AstraZeneca has a number of local Graduate/Management Trainee Programmes available. Search by country on our global careers website, or follow the links to explore careers on your local AstraZeneca website.
Operations Graduate Programmes
Locations: China, Sweden, UK, US, Mexico
We’re looking for open-minded, forward-thinking individuals eager to drive innovative change and influence our future direction of travel. Take the initiative, own it and run with it to make a difference for patients and our business. All while strengthening your capabilities and gaining a valuable insight into our broad business. Whether you’re looking to pursue a particular passion or are still figuring it out, the diverse experiences and transitions in the Operations Graduate Programmes will help you shape your future, no matter which path you follow.
Pharmaceutical Technology & Development Graduate Programme
Locations: Macclesfield, UK & Gothenburg, Sweden
Tackle new scientific challenges. Seek opportunities to learn and grow in a highly supportive team. Collaborate and build close connections with peers and experts across the business. Join our PT&D graduate programme and enjoy a high level of emphasis on your personal development and career progression. Work on a variety of projects aligned to your experience and career ambitions. Build a broad skill base that will enable you to strengthen both your managerial and technical capabilities. And accelerate your learning.
Precision Medicine Academy
Precision Medicine is at the heart of what science can do at AstraZeneca. It combines the expertise of specialists from across the organisation, unlocking new potential approaches that put patients first, setting us apart from our competitors. Through advances in diagnostic testing, we are now able to identify how unique individuals respond to treatments, and match them with medicines that deliver the best outcome for them. Since 2014, we've been ranked among the top pharmaceutical companies for precision medicine approvals - proof of our world-class practices.
R&D Graduate Programme: Early Phase Drug Discovery
We’re looking for the next wave of scientists to make an impact and influence the agenda. Join our R&D programme and bring your skills, drive and curiosity to our teams and projects. Thinking innovatively and taking the initiative to help drive our drug design and development. Completing novel and cutting-edge research in state-of-the-art laboratories and developing the knowledge and confidence to become the scientist you aspire to be.
Technology Graduate Leadership Programme
Locations: India, Spain & Mexico
Our IT team is at the forefront of discovering and driving digital health and workplace automation to ensure our teams can continue to develop life-changing medicines. As a member of our Technology Graduate Leadership Programme, you’ll join our next wave of technology leaders. Use your skills and influence to deliver technology solutions. Improve the way we operate, serve customers and enable our teams to do their best work. And enjoy being recognised for your knowledge, experience and ideas. Together, we’ll drive our technology capabilities and make meaningful impact for our business and patients.
Clinical Research Associate (CRA) Graduate Programme
Location: Wilmington, DE
Designed to prepare you for a career as a Clinical Research Associate, you’ll progress through different programme rotations, gaining valuable experience across functions within Site Management and Monitoring and building multidisciplinary skills. Supported by experts in their field, you’ll receive mentoring and training, bringing limitless opportunities to learn, grow, and develop your strengths in different ways.
Commercial Leadership Development Programme (MBA)
On this rotational programme, you’ll have the ability to learn and grow across global functions, markets and our broad pipeline of products. Expanding your knowledge and developing a global mindset to take you to the next level. Collaborating with bright, diverse minds across the business. There’s never been a better time to be part of our journey – at the cutting edge of innovation, impacting millions of people around the world. Explore our transformational opportunities and accelerate your development.
AstraZeneca's Graduate Programmes are open to Masters level applicants. Applicants with an MBA should consider our Commercial Leadership Development Programme.
Search "AstraZeneca" on www.findaphd.com to explore any PhD studentships we are currently offering in partnership with our academic collaborators.
Postdoc Programme
Locations: UK, US and Sweden
Our Postdoc Programme is for self-motivated individuals looking to take on interesting, high-impact projects in a collaborative, challenging and reputable environment. As a respected specialist, you’ll have the freedom and autonomy to contribute known skills, and the support to rapidly learn new approaches to follow the science, innovate and make an impact. Leveraging scientific development to springboard your career whilst making a meaningful difference to patients, science, and our business. Collaborating and sharing knowledge cross functionally, together with independently leading projects in your field of interest.
R&D Postdoctoral Challenge
The R&D Postdoctoral Challenge invites final year MD and/or PhD students and Postdoctoral researchers to propose innovative ideas to transform the treatment of some of the world's most complex diseases. This exciting opportunity offers a fully funded postdoctoral position, where you will join our vibrant scientific community to conduct pioneering research within one of AstraZeneca's core disease areas or expert functions. You will have access to the expertise, compounds, novel tools and technologies, and mentoring support you need to turn your ideas into reality.
Why choose AstraZeneca?
Starting your career with us means following the science, boldly disrupting norms and supporting one another in shaping the future. Collaborate with leaders and teams across a wide range of disciplines and explore your passions. Discover your career purpose with us. And build strong bonds that will last a lifetime.
We discover, design, develop and deliver life-changing medicines. Our teams deliver innovations across all areas of our business – from diabetes care to genetics. With career paths spanning the medical lifecycle, you could be joining us in areas like Clinical Development, Data Science and AI, Finance, Manufacturing or Supply. Explore our opportunities and kickstart the career that could take you from emerging professional to renowned subject matter expert.

Our Early Talent Awards
People are our top priority – our patients, colleagues and communities. Regardless of what programme you’re in, we bring diverse talent from all over the world together. We enable international perspectives and combine our strengths and knowledge to multiply our impact. That’s why we’ve won multiple awards as a trusted Biopharmaceuticals employer. These awards show us that we’re on the right track and they’re an incentive to work even harder on nurturing our culture and making it ever richer and more diverse.

Writing the perfect CV
Recall experiences in which you've demonstrated the behaviours listed in the job description
Highlight your contact information and make sure your voicemail message is clear
Make pertinent information like education, professional experience, leadership roles, team participation and volunteer experience easy to find
Format your CV so it's readable and concise. One page is preferable but two makes sense if you’ve got a lot of experience
Use descriptive language to present your experiences
Have evidence and examples to support your statements
Wordiness. Use short sentences. Treat your CV like an advertisement
Excessive use of borders, italics and fancy fonts. They don't upload cleanly into online systems
Overly small font size. Stick to 10-12 point
Too much bold type. Only enbold major achievements
Navigating the Application Process
Discover detailed insights and get on an AstraZeneca recruiter's short list with our application hints and tips.


We’ll keep you up to date
Sign up to be the first to receive job updates
Email Address
Confirm Email
By signing up, I acknowledge I have read the AstraZeneca privacy policy , and I wish to receive email communications and SMS communications. I understand I can opt-out from receiving email and SMS communications at any time.
We are AstraZeneca, one of the world’s most forward-thinking and connected BioPharmaceutical companies. Explore our world.
At AstraZeneca, our purpose is to help patients all over the world by delivering life-changing medicines as one collaborative team.
Inclusion & Diversity
The success of AstraZeneca is founded on innovation, creativity and diversity. Discover what this means for you.

Great culture, great work assignments, supportive management. Rotation opportunity within the company. They value inclusion and diversity.
Fraud Alert
It has been brought to our attention that there has been fraudulent activity by scammers attempting to represent themselves as Parexel employees or recruiters. These individuals are attempting to reach potential job seekers through online chat interviews and sending false offer letters, representing Parexel without our consent. If you’re concerned that you’ve been contacted by an unauthorized Parexel recruiter or employee, please notify [email protected] . You may also report suspicious fraudulent activity to your local law enforcement agency. Thank you.

- Global Careers
- China Careers
- Japan Careers

I’m working toward a better future for patients. And I do it
Search Jobs
Radius: Enter distance 5 15 25 35 50
Begin your career journey at Parexel
We hire emerging talent and current students into a variety of positions across all regions and business units. You will be at the heart of getting medicines to those who need them most.
Intro/Headline Here
At Parexel you will be part of a team dedicated to accomplishing great things together; you will learn how we take personal responsibility for making success happen, and how individual skills add value to our collaborative culture.
Set the foundation for your professional development and career growth. Gain insight into one of the leading global Clinical Research Organizations with our entry-level positions and develop your own scientific profile with our emerging talent programs, internships, placements and apprenticeships.
Our Emerging Talent Programs
Apex opportunities.

PharmaTimes Clinical Researcher of the Year Award – Americas winners
Jake Andary, an APEX CRA graduate earned the GOLD Award in the Novice CRA category
Maura Hughes and Sona Sran, two APEX CRA graduates named finalists
See LinkedIn post
The Accelerated Program of Education, Exposure, and Experience for CRAs (APEX CRA) provides foundational clinical research training - including project and system experience - to new CRA hires who are recent college graduates and have less than a year of monitoring experience.
Our APEX Program hiring cycles, for both AMER and EMEA, are on a regular cadence which aligns with our business needs. At various times we might not be actively recruiting as we are in-between hiring cycles for the next class. Please check this site for regular updates as all open and available APEX CRA position locations are posted here

Ryan: Summer 2022 Program
“I learned many different skills from the APEX CRA Program! Ranging from TMF maintenance to how to properly conduct various visit types, but the most important thing I learned was to remain confident in whatever task I was completing. The APEX CRA Program gave me the proper tools to confidently complete my visits and find resolutions to issues at my site.”

Jake: Spring 2023 Program
"The APEX CRA Program is an excellent platform to further build your clinical research experience and launch you into the clinical research associate role. You will be given support along the way to continue honing your CRA skills and solidify a strong foundation for the rest of your clinical research career."

Mimi: Fall 2022 Program
"The thing I enjoyed most about the APEX CRA Program was meeting others that were on the same learning journey as I was. To this day, we keep in touch and work through new issues we experience as CRAs. There is a sense of comfort in knowing that you are not alone in learning the job."
Path to Project Leader Program

Ali Hamidi , a Path to Project Leader graduate named a finalist
The Path to Project Leader Program provides the opportunity for highly talented, motivated, and quickly progressing candidates to join the clinical research industry in a Senior Project Specialist role to acquire deep clinical research and project management knowledge and progress to an Associate Project Leader role within eight months .
Currently hiring:
June 2025 Cohort Remote - United States Applications due January 1, 2025
Apply now Watch the video to receive:
- An overview of the program (0:25)
- How do Pharmacists bring value to Clinical Research (2:55)
- What is their experience with the program? (3:40)
- Why should you join Parexel? (6:00)

Our Internships / Apprenticeship / Entry-Level Opportunities
At Parexel, our placement opportunities, internships and apprenticeships offer a unique chance to actively contribute to life-changing work and start your career on a path with purpose. With options available globally across various departments, you can find your perfect fit no matter the time of year.
Explore where your professional interests align with our purpose-driven work and let’s make a difference together! Hiring for different programs kick off throughout the year based on business needs.
Associate Data Engineer
You will be involved in designing, building, and maintaining data pipelines using Microsoft Azure services, with a focus on DataBricks and PowerBI in a medallion architecture. The role encompasses the entire software development lifecycle, including prototyping, coding, testing, performance optimization, and collaboration with various teams for deployment and issue resolution.
United States - Durham - North Carolina *Must be legally authorized to work in the US without the need for visa sponsorship now or in the future*
Clinical Database Programmer
You work with close supervision to support various programming activities related to clinical systems, and/or the application of computer and communication technologies.
Based in Sheffield, UK or Bloemfontein, South Africa
Placement Students in Sheffield, UK This is a 12 -14-month University Placement opportunity for students in their penultimate year of study. The placement will begin Summer 2025 and be based in our Sheffield office.
Statistical Programmer
You will work with an assigned mentor to help with your training and development. Duties would include producing datasets, working on statistical outputs such as statistical tables and graphs, and data listings, and utilizing SAS® Software.
Based in Sheffield, UK
Clinical Data Engineer
You work with close supervision to support various activities including managing data aggregation from various external sources and producing listings, reports and insights utilizing SAS® Software or the Data Management Workbench platform.
Biostatistician
You will support an experienced Biostatistician. Key responsibilities include producing data listings using efficient programming techniques, conducting statistical analyses under supervision, and creating statistical reports.
The Finance Department offers 12-week Summer Internships for roles like Financial Analyst, Contract Specialist, and Corporate Services.
Based in Durham, North Carolina, USA (hybrid)

Senior Manager, IT Security and Manager of U.S. Army Veteran, Thomas
"Thomas joined Parexel as part of Data and Technologies' inaugural internship program this past Summer. From day one, he demonstrated a positive attitude, an eagerness to learn, and a commitment to excellence. His drive and dedication quickly set him apart, making the decision to offer him a full-time position an obvious choice. Thomas's internship experience allowed him to transition seamlessly into his full-time role, where he has already made a significant impact on our team. His ability to quickly adapt and contribute has been impressive, and we are excited about his potential in the technology and cybersecurity space. We look forward to witnessing Thomas's continued growth and future accomplishments at Parexel!"

Bella: Corporate Sustainability Analyst (Former Intern)
“I wanted to work for a company with both a strong mission and values, so continuing my career at Parexel felt natural. My Corporate Service Internship set me up for success and made me feel confident that Parexel was somewhere I could envision myself working. I also chose Parexel because there is a lot of discovery in how to make clinical trials more sustainable and I want to play a part in the progress being made.”

Thomas: IT Security Analyst (Former Intern)
"I would say the two most important things I learned during my IT internship were how to better work with and use AI and how to use different cybersecurity tools to properly identify and triage any malicious activity. Everyone here at Parexel is very kind and welcoming. Specifically for the IT field, there seems to be a lot of opportunity and so far, my job has been very rewarding."

Danielle: Statistical Programmer I (Former Intern)
"Parexel took a chance on me when I had minimal experience and has given me the chance to grow exponentially. What I enjoy the most is the fact that I get to work in a team that makes a difference. Finding the solution to a problem and using independent thinking and strategy to achieve the overarching goal is the best part of working in the clinical industry, for me.”
Why Parexel Is a Great Place to Work
At Parexel, we define ourselves by the difference we make and by the compassion we share while making it. We advance science by keeping the patients at the heart of everything we do. We believe in each other, and that allows us to accomplish great things ourselves.
Work that puts patients first
Work that accelerates inclusive clinical trials
Work that helps you grow
Work that values collaboration
Work that gets rewarded
Work that embraces flexibility
Tips and tricks for your application.

Antoinette's Tip:
Know how to communicate your strengths.
Watch Video

Isabella's Tip
Be open, flexible & pay attention.
Get in touch with our Emerging Talent Recruitment team

Amy van Kirk Senior Director, University Recruiting

Pavandeep Mann University Programs Manager

Jordan Teagarden Talent Advisor - Emerging Talent

Antoinette Bassett Talent Advisor - Emerging Talent

Isabella Moore Recruitment Coordinator
Our Emerging Talent Events
During long-standing connections with universities around the world, we recruit top talent at several events throughout the year. You can meet us at University Recruitment Fairs, Diversity Hiring Events, and at the Boston Career Forum.
Check out our current events:
Explore Parexel
Patients first – Parexel employees share how they work with the patients in mind.

Our work culture
Learn about our culture, perks, learning opportunities, and our corporate responsibility approach.

About our hiring process
It’s all about finding the right fit, for you and for us. Our recruiters work with you and our hiring managers to bring together a team and culture where everyone can grow and be successful.
Diversity, Equity & Inclusion at Parexel
Learn how Parexel embraces diversity and strives for equity and inclusion in its workforce, clinical trials, and supplier partnerships.
JOBS FOR YOU
There is no jobs to view
You currently have no saved jobs to view.
Make sure to accept the Functional cookies to enable this module to display saved job links. You will be able to review them here when you revisit this page..
View Open Positions
Sign up for our Talent Community
Sign up and we’ll reach out with job alerts when positions that match your career interests become available. We’ll also share periodic updates about the latest company news and events.
- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries
The Pathway to Becoming a Clinical Research Associate
Introduction to a Clinical Research Associate Career
The role of a clinical research associate, essential skills for a successful cra, certification and education requirements, clinical research coordinators (crc) and clinical trial assistants (ctas), entry-level cra positions, career progression in research, clinical trial project manager (pm) or clinical trial manager (ctm), clinical trial it specialist, conclusion and future trends in cra careers.
The clinical research enterprise supports the discovery of novel therapies, interventions, and diagnostics in many therapeutic areas and applications. Driven by technological enhancements and gains in precision medicine, clinical research professionals are in high demand. Speculative reports indicate the clinical trial market will reach over 70 billion dollars by 2028. [1] With concerns over the anticipated shortages of clinical research associates (CRAs), there are many training options available to choose from.
CRAs can expect to make between 60K and 120K+ per year, depending on a combination of experience, job requirements, location, and education. Individuals may seek employment opportunities from pharmaceutical companies, contract research organizations (CROs), and the federal government. Since they are part of the enterprise of improving patient outcomes, most find their careers in clinical research highly rewarding.
The primary work of a clinical research associate (CRA) consists of monitoring the activities of a clinical research site. Their scope of responsibility also includes facilitating communications between sponsors and research sites, ultimately helping a clinical study reach a successful completion.
A CRA is tasked with overseeing the implementation of a clinical trial protocol in a manner that prioritizes patient safety and data integrity. Specific activities include monitoring:
- Subject recruitment
- Subject screening and enrolment
- Adherence to Good Clinical Practice
- Generation of complete and accurate documentation
- Recording of study data into case report forms and electronic data capture systems
- Study site management
CRAs serve as the nexus among key study stakeholders, such as pharmaceutical companies, principal investigators, clinical research coordinators (CRCs), and clinical data managers. They are core facilitators in the production of the scientific data essential to the development of life-saving diagnostics and therapeutics. [2]
Becoming a successful CRA typically begins with an interest in clinical research. It is common for individuals who enjoy fast-paced work environments, critical thinking, and helping others to choose a research career.
Successful CRAs demonstrate above-average communication, problem-solving, and organization skills. Industry veterans also tend to display a natural tendency towards empathic-based leadership qualities and the ability to work well under pressure.
The CRA is often in charge of multiple study sites. Subsequently, a clinical study professional should be comfortable with travel and demonstrate a high level of computing proficiency to manage substantial amounts of reporting and documentation.
Complementary skillsets that can contribute to the success of a CRA include:
- A comprehensive understanding of activities related to early drug discovery through commercialization
- Advanced medical or scientific knowledge because study data features complex medical terminology, statistical elements, and medical procedures
- Advanced writing abilities to handle the high density of communications
- Well-developed project management skills
Most CRA positions require a bachelor’s degree in life sciences or related fields and a healthy amount of clinical study experience. Several universities offer graduate certificates or master’s degrees in clinical research or clinical trial management. Obtaining a CRA university credential consists of meeting specific eligibility benchmarks determined by educational background, hours of CRA-related experience, letters of recommendation, and industry-specific training records.
Two industry associations offer professional credentials through certification exams. In order to sit for the exam, an aspiring CRA needs to have completed extensive hours working in the field.
The roadmap to obtaining a CRA certification from the dominant professional society mandates the following requirements and benchmarks [3]:
- A minimum of a bachelor’s degree in a life science-related discipline and 3,000 hours of work in human subjects research
- Or an undergraduate or graduate degree (excluding graduate certificates) in the field of clinical research plus 1,500 hours of work in human subjects research
- And an advanced knowledge of applicable regulations, Good Clinical Practice guidelines, and HIPAA requirements
It should be mentioned that professional association credentialing is not necessary to work as a CRA, although some find certification helps obtain employment in certain circumstances.
Career Pathways and Advancement
As with many chosen career paths, the journey to becoming a CRA is not necessarily linear. Many successful CRAs find that training as a CRC allows for greater industry experience and a more fluid transition into a prominent CRA role. A CRC, and sometimes a research nurse, helps conduct a clinical trial and typically works at one site. The transition to a CRA requires a shift in perspective from one who conducts the trial to one who monitors those who conduct trials at several sites.
Alternatively, CRAs may begin their careers as clinical trial assistants (CTAs). CTAs assist with eligibility assessments, personnel management, and participant needs. They also oversee some of the clinical site’s daily functions, which may include correcting safety hazards, maintaining HIPAA and compliance records, and ordering supplies.
Entry-level CRA positions provide a fantastic opportunity to gain more field experience. To help hone their understanding of what is expected, it is common for entry-level CRAs to work alongside other senior members or clinical trial leads.
Hallmarked by extensive administrative and scheduling duties, research data generation and recording, subject recruitment and retention, and determination of study eligibility, entry-level CRAs should learn all the functions that coincide with a clinical trial. Other job functions entail understanding the U.S. Food and Drug Administration’s (FDA) regulatory requirements for clinical research and product market approval.
There are many lucrative management-level opportunities for senior-level CRAs. Most positions require a minimum of a bachelor’s degree. However, some higher-paying jobs may require candidates to have a master’s or doctoral degree, along with a strong record of accomplishment and qualifying experience. CRAs who enjoy mentoring others may also find that exploring training-type positions may provide new opportunities.
Related Career Opportunities in Research
A person in one of these roles is in charge of planning and overseeing the functional details of clinical trials. Working directly for a pharmaceutical company sponsor or CRO, a PM or CTM organizes the personnel, resources, and conduct of research, from initial trial design through study closeout and the final analysis of trial data. The PM or CTM is also responsible for study site management, data capture, analytics, monitoring, validation, and reporting results.
Pivotal to the applications needed to fuel the trend toward decentralized clinical trials, the clinical trial IT specialist is responsible for maintaining the systems used to conduct participant activities and gather study data. This includes optimizing user interfaces for digital functionality and multi-device capabilities. The IT specialist is often responsible for overseeing the integration of electronic data capture systems and maintaining systems used for clinical data analysis. The clinical IT specialist also represents the first line of defense against patient privacy violations, system threats, and HIPAA violations. [4]
Fueled by the need for improved outcomes, we can expect more innovations to emerge from the research sector in the years ahead. There is also a growing momentum to bring more inclusive trial experiences to marginalized populations. Catapulting off an already robust climate, the demand for clinical research professionals will increase significantly. The high demand could dictate the way that the clinical trial industry approaches staffing decisions by hiring based on specific proficiencies.
Current trends hint at a bolder convergence between real-world data and artificial intelligence that may result in improved predictive disease modeling, clinical decisions, and drug optimization. [5] Algorithmic-based capabilities also carry the potential to inform recruitment strategies by removing some of the barriers that impede participation, thereby improving patient diversity.[6] These trends will shape the future of CRA work, thereby demanding that CRAs stay abreast of changes in how clinical trials are designed and implemented.
With so many revolutionary changes to the medical and clinical trial landscape, there has never been a more exciting time to join the research community. Learn more about CRA opportunities today.
1. Markets and Markets Clinical Statistics. Clinical Trials Market Statistics . Accessed June 17, 2024
2. Kandi, V., & Vadakedath, S. (2023). Clinical Trials and Clinical Research: A Comprehensive Review . Cureus, 15(2).
3. Association of Clinical Research Professionals. “ ACRP-CP Certification .” Accessed June 17, 2024.
4. He Y, Aliyu A, Evans M, Luo C. Health Care Cybersecurity Challenges and Solutions Under the Climate of COVID-19: Scoping Review . J Med Internet Res. 2021 Apr 20;23(4): e21747.
5. Harrer, Pratik Shah, Bhavna Antony, and Jianying Hu. Artificial Intelligence for Clinical Trial Design . Trends in Pharmacological Sciences 40(8):577-591. Accessed June 17, 2024
6. Kurt, A., Semler, L., Meyers, M. et al. Research Professionals’ Perspectives, Barriers, and Recommendations Regarding Minority Participation in Clinical Trials . J. Racial and Ethnic Health Disparities 4, 1166–1174 (2017)

- On Campus Podcast – Supporting Undergraduate Student Success and Retention
- What CITI Program is Reading – December 4, 2024
- CITI Program Media Playlist – November 14, 2024
- Enhancing NIH Grant Management Operations with AI and Digital Technology
- Research Compliance Coordinator III
- IBC Assistant Director (hybrid work assignment)
- IRB Analyst I or II
- Human Research Protection Program Director

CRA Careers at IQVIA
Help tackle healthcare’s greatest challenges to improve patient health.
Not ready to apply? Join our Global Talent Network .
Put your passion to work
IQVIA Clinical Research Associates play a vital role in the evolution of clinical development. They bring passion, ambition, and a deep level of expertise to help solve complex clinical issues while ensuring adherence to regulations and sponsor requirements. Here, you'll find the autonomy and flexibility you need to take your CRA career to the next level.
Choose your path
Sponsor-dedicated.
When you join as a sponsor-dedicated CRA, you’ll embrace the stability of a global contract research organization and harness the power of industry-leading technology. As part of this alignment, you'll gain valuable experience, working side by side one of our pharmaceutical customers, and expand your knowledge in a wide variety of therapeutic areas.
Our sponsor-dedicated model brings together the right people, experience and ideas to deliver high quality solutions specific to the customer’s needs.

Full-service
When you become a CRA as part of our full-service alignment, you'll embrace your passion for a specific therapeutic area and support a variety of sponsors. With access to world-class training and cutting-edge technology, developed specifically for IQVIA CRAs, you'll have the resources you need to create the career you want.
Our full-service model delivers cutting-edge clinical research through state-of-the-art innovation.

IQVIA Biotech
When you pursue a CRA career with IQVIA Biotech, you'll work directly with customers in a collaborative environment to help change the face of biotech. Using your extensive therapeutic knowledge, you’ll oversee uniquely-focused clinical studies, where you’ll be exposed to pioneering protocols and experience a dedicated partnership with your team like none other.
IQVIA Biotech is at the forefront of clinical trials in a variety of therapeutic areas through the delivery of flexible solutions within the biotech sector.

IQVIA empowers you to drive your own path within the organization. As no person's route is the same and the clinical research landscape is forever changing, you are encouraged to continually seek career growth opportunities and align your interests with your career goals.

Senior Clinical Research Associate 2
At IQVIA, we are committed to supplying our CRAs with the right tools, training and development support to allow for success and career growth. In addition to the rewarding work, collaboration and engaged line management, there is a constant focus on development as your career growth supports also the growth of the company.

Director, Clinical Operations
For me, it’s the people and knowing I’m making a difference in creating a healthier world that inspire me to come to work every day. I can go to anyone with a challenge, and they are willing to help. Together, we’re thinking outside of the box to navigate each sites’ unique needs and challenges.

Senior Clinical Research Associate
Explore unlimited opportunities
Whether you choose to advance within a specific therapeutic area or want to explore something new, our award-winning CRA Training Program encourages you to pursue your unique passions and create the career you want. Explore the opportunities available when you join IQVIA as a CRA.
What you can expect
All CRAs at IQVIA, regardless of alignment, thrive within our dynamic culture and experience:
Professional Development

Work-Life Balance
Supportive Leadership

Best-in-Class Training

Collaboration
What sets us apart, quick questions with a cra in amsterdam, quick questions with nisha shah, cra career in australia and new zealand, explore clinical monitoring jobs.
- R1447543 Senior Clinical Research Associate (m/w/d), Single Sponsor Learn more Multiple Locations
- R1411039 Senior Clinical Research Associate - Belgium (home-based) Learn more Multiple Locations
- R1441583 Clinical Research Associate, Site Management - Italy Learn more Multiple Locations
Join our Global Talent Network
Let’s stay connected. Sign up to receive alerts when new opportunities become available that match your career ambitions.
Job Category Select a Job Category Administrative Support Advanced Analytics Artificial Intelligence & Machine Learning Business Systems Analysis Client Services Clinical Data Management Clinical Operations Clinical Project Management/Leadership Clinical Trial Supply & Logistics Connected Devices Consulting Contract Management Database Management Systems Epidemiology Finance General Management Human Resources Information Security Internships IT Design & Development IT Infrastructure IT Support Lab Science Lab Science Advisors Lab Services Laboratory Laboratory Projects Legal and Regulatory Lifecycle Safety Marketing Medical Medical Affairs Medical Communications Medical Sales & Services Monitoring Patient Centric Services (PCS) Phase 1/Clinic Operations Product Support (Tier 3) Production Project and Program Management QA & Testing Quality Assurance Safety & Security Sales Sales Support Software Development Engineering Statistician Strategic Supplier Services Strategy and Corporate Development Technical Writing
Remote Select... Yes
Confirm Email
Search for a location and select one from the list of suggestions. Select a job category from the list of options. Finally, click “Add” to create your job alert. Multiple job alerts may be created by repeating these steps.
Explore life at IQVIA

IMAGES
VIDEO
COMMENTS
To be eligible for the program, you'll need: A bachelor's or master's degree in life sciences or a clinical research field. No more than two years' post-graduate work experience. Strong organizational, communication, and collaboration skills. A passion to work in pharmaceuticals, with a real desire to make a difference to patients.
Sometimes knowing how, and where, to start your Clinical Research Associate (CRA) career can be challenging.That's why our industry leaders established Parexel's Accelerated Program of Education, Exposure, and Experience (APEX) for CRAs back in 2022.The goal of the APEX Program is to provide foundational clinical research training - including project and system experience - to individuals ...
What They Do: Clinical Research Associates assist in laboratory, survey and other science research, prepare findings for publication, manage databases, ... For the academic year 2024-2025, students enrolled in an online graduate academic program will be charged a graduate online program fee of $125 per year.
What is a Clinical Research Management Graduate Program? The online Master's in Clinical Research Organization and Management degree is a 36-semester credit (12 course) non-thesis fully online program. ... To be ACRP certified, a Clinical Research Associate is expected to have proficiency in the six (6) main content areas of clinical research ...
The Bachelor of Science in Health Sciences degree from George Washington University is a 120-credit program that's available entirely online. As part of this clinical research associate degree online, you'll take 17 core courses worth 51 credits. You must also take nine credits of electives in clinical research for a total of 60 credits ...
Clinical Research Associate (CRA) Graduate Programme Location: Wilmington, DE Designed to prepare you for a career as a Clinical Research Associate, you'll progress through different programme rotations, gaining valuable experience across functions within Site Management and Monitoring and building multidisciplinary skills.
Spring 2023 Program "The APEX CRA Program is an excellent platform to further build your clinical research experience and launch you into the clinical research associate role. You will be given support along the way to continue honing your CRA skills and solidify a strong foundation for the rest of your clinical research career."
MSCR MS Program. The MSCR MS Program, leading to a Master of Science in Clinical Research graduate degree, is designed to develop physician scientists interested in clinical research or biomedical informatics to:. Design and conduct clinical research (clinical trials and observational studies) Successfully compete for funding (e.g., foundation grants, NIH K23, or R01's in clinical research)
Several universities offer graduate certificates or master's degrees in clinical research or clinical trial management. Obtaining a CRA university credential consists of meeting specific eligibility benchmarks determined by educational background, hours of CRA-related experience, letters of recommendation, and industry-specific training records.
IQVIA Clinical Research Associates play a vital role in the evolution of clinical development. They bring passion, ambition, and a deep level of expertise to help solve complex clinical issues while ensuring adherence to regulations and sponsor requirements. ... IQVIA's CRA Training Program: Where possibilities are limitless Virtual learning ...