An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective
Paolo sambo, carlo nicoletto, andrea giro, fabio valentinuzzi, tanja mimmo, paolo lugli, guido orzes, fabrizio mazzetto, stefania astolfi, roberto terzano, stefano cesco.
- Author information
- Article notes
- Copyright and License information
Edited by: Victoria Fernandez, Polytechnic University of Madrid, Spain
Reviewed by: Md Asaduzzaman, Bangladesh Agricultural Research Institute, Bangladesh; Maria Del Carmen Salas, University of Almería, Spain
*Correspondence: Stefano Cesco, [email protected]
This article was submitted to Plant Nutrition, a section of the journal Frontiers in Plant Science
Received 2019 Apr 10; Accepted 2019 Jul 1; Collection date 2019.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Soilless cultivation represent a valid opportunity for the agricultural production sector, especially in areas characterized by severe soil degradation and limited water availability. Furthermore, this agronomic practice embodies a favorable response toward an environment-friendly agriculture and a promising tool in the vision of a general challenge in terms of food security. This review aims therefore at unraveling limitations and opportunities of hydroponic solutions used in soilless cropping systems focusing on the plant mineral nutrition process. In particular, this review provides information (1) on the processes and mechanisms occurring in the hydroponic solutions that ensure an adequate nutrient concentration and thus an optimal nutrient acquisition without leading to nutritional disorders influencing ultimately also crop quality (e.g., solubilization/precipitation of nutrients/elements in the hydroponic solution, substrate specificity in the nutrient uptake process, nutrient competition/antagonism and interactions among nutrients); (2) on new emerging technologies that might improve the management of soilless cropping systems such as the use of nanoparticles and beneficial microorganism like plant growth-promoting rhizobacteria (PGPRs); (3) on tools (multi-element sensors and interpretation algorithms based on machine learning logics to analyze such data) that might be exploited in a smart agriculture approach to monitor the availability of nutrients/elements in the hydroponic solution and to modify its composition in realtime . These aspects are discussed considering what has been recently demonstrated at the scientific level and applied in the industrial context.
Keywords: nutrient acquisition, biofortification, nutrient interaction, plant growth-promoting rhizobacteria, nanoparticles, sensors, smart agriculture
Introduction
Considering that human world population will reach about 9 billion by the year 2050 ( Tilman et al., 2002 ), it appears clear that food security is one of the pivotal themes of the new millennium and, reasonably, the most urgent challenge for the agricultural sector. However, it should be considered that the progressive drop of fertile soil surface, due to environmental pollution and urbanization phenomena ( Chen, 2007 ), greatly complicates the context. In this regard, the intensification of the production cycles and the monoculture approach, which favored the diffusion of many pathogens and the development of the corresponding pathologies ( Jones et al., 1991 ; Shafique et al., 2016 ; Tittarelli et al., 2016 ), should also be taken into account. Moreover, the strict dependency of agricultural practice on water availability ( Verones et al., 2017 ) in an age of drastic climate changes (desertification) makes the scenario even more complex. In this respect, the possibility of exploiting surfaces not anymore fertile (due to pollution or pathogen problems) for agricultural purposes and also limiting at the same time the water consumption (see also Table 1 ; Pignata et al., 2017 ; Müller et al., 2017 ) makes the soilless system cultivation surely a valid opportunity. Moreover, it should be highlighted that this cultivation approach also represents a favorable response toward a more environmentally friendly agriculture ( Benke and Tomkins, 2017 ; Maucieri et al., 2017 ; Tajudeen and Taiwo, 2018 ) as well as a promising tool also in the vision of the general challenge of food security.
Water use efficiency (WUE) in soilless and soil cultivation systems of several crops.
Values were estimated using FAO references ( http://www.fao.org/3/u3160e/u3160e04.htm ) adopting a sub-humid cultivation area and an average ETo of 5 mm day −1 . The following formula was used to calculate the crop evapotranspiration: ETc = [(kc × ETo) × average length of total growing season (days)] where ETo is the reference crop evapotranspiration and kc is the crop factor. kc values were used according to Dukes et al. (2012) .
Currently, about 3.5% of the worldwide area cultivated under tunnels and greenhouses for vegetables production adopts the soilless agriculture techniques based on hydroponic solution (such as floating systems, nutrient film technique (named also NFT) – or aeroponics, Hickman (2016) ). This significant diffusion at the field scale undoubtedly highlights the presence of many advantages of this production approach in addition to the more efficient use of the nutritional resources including water ( Kinoshita et al., 2016 ; Rodriguez-Ortega et al., 2017 ). In fact, there is a variety of examples where hydroponic solution can be efficiently used for biofortification programs with oligo elements (e.g., iodine (I), selenium (Se), silicon (Si), and calcium (Ca), see also Table 2 ; Schiavon et al., 2013 ; Tomasi et al., 2013 , 2015a , b ; Smoleń et al., 2016 ; D’Imperio et al., 2016a , b ; Mimmo et al., 2017 ) as well as to improve vegetable quality and its shelf life according to the market and consumer needs ( Giuffrida et al., 2014 , 2017 ; Amalfitano et al., 2017 ; Giro and Ferrante, 2017 ; Yasuor et al., 2017 ; Islam et al., 2018 ). However, the extensive research activity aimed at fine-tuning fertilizers’/nutrients’ concentration in the hydroponic solutions, particularly (1) to restrain nitrate content in edible plant tissues ( Fageria, 2010 ), (2) to guarantee vegetables’ safety, and (3) to improve the nutritional quality of the yields ( Fallovo et al., 2009 ; Gruda, 2009 ; Putra and Yuliando, 2015 ), point out that there is still much room for improvement. This is particularly true in the light of the recent scientific pieces of evidence about the mechanisms underlying the mineral nutrition in plants and their regulation (competition/antagonism/interaction among nutrients) and about the bio-geochemical cycles of nutrients in the soil solution (solubilization/precipitation). This knowledge is fundamental when the development of different strategies (including approaches and tools such as beneficial microorganisms-PGPRs and nanoparticles) for the optimization of the hydroponic production of vegetables is pursued.
Mineral biofortification in soilless vegetables species (research activities are represented by coloured cells).
In the industrial context, a new paradigm labeled as Industry 4.0 or Smart Manufacturing and based on cyber-physical manufacturing systems and Internet of Things is emerging ( Kagermann et al., 2013 ). It is interesting to note that hydroponic production of vegetables carried out in limited and well-controlled environments is going to reduce the most relevant differences between agricultural and industrial processes and, in turn, to improve the quality control. However, to achieve this goal, a set of additional monitoring tools specifically for the hydroponic-based cultivation approach are necessary. In this regard, sensors for realtime monitoring of the hydroponic solutions composition (i.e., availability of nutrients/elements) as well as interpretation algorithms also based on machine learning logics to analyze such data play a pivotal role. In fact, only thanks to these tools, that can be borrowed from the industrial context, it might be possible to maintain/adapt in realtime the composition of a hydroponic solution in order to achieve products of a desired quality.
This review is aimed at analyzing the open questions of hydroponic systems and at highlighting opportunities in their applicative use in a field scale, considering also what has recently been demonstrated at the scientific level and applied in industrial context. In particular, from the scientific point of view, we would like to unravel the topics and problems that have been adequately studied and the ones that instead still require significant research efforts, all these pieces of information being essential for a better management of crop nutrient acquisition in soilless systems. Moreover, from the practical point of view, the potential to use new forms of nutrients and/or bioeffectors as well as new technologies to collect/analyze data could be a viable tool at the field scale for farmers in a context of a smart agriculture.
Open Issues of Hydroponic Solutions Used in Soilless Agriculture Techniques
It is widely known that the productivity and quality of crops grown in hydroponic systems are markedly dependent on the extent of the plant nutrients acquisition from the growing medium ( Valentinuzzi et al., 2015 ). It is interesting to highlight that this root physiological process is not only affected by the availability levels of the nutrients (i.e., by their soluble forms) in the medium (solubilization/precipitation: section “Chemical Management of Nutrient Availability in the Hydroponic Solution”), but also by the nutrient sources (nutrient chemical forms: section “Nutrient Chemical Forms and Uptake Processes”) and/or by the interactions among the different nutrients (e.g., competition/antagonism: section “Nutrient Interactions”).
Chemical Management of Nutrient Availability in the Hydroponic Solution
When dealing with hydroponic cultures, solution chemistry is fundamental to ensure adequate nutrient concentrations for plant uptake. In particular, multiple chemical equilibria must be taken into account when preparing nutrient solutions using salts or concentrated liquid stocks, especially solubilization/precipitation equilibria ( De Rijck and Schrevens, 1998b ). In fact, a number of physical-chemical phenomena can alter the nutrient availability for plants, the most important of which are (1) precipitation, (2) co-precipitation, and (3) complexation. In this respect, it should be highlighted that the temperature of the nutrient solution, affecting the chemical equilibria in solution, may considerably influence these processes. This is particularly crucial for areas where the overwarming of the nutrient solution often occurs, impacting also at the physiological level of crops ( Lee and Takakura, 1995 – spinach; Fazlil Ilahi et al., 2017 – butterhead lettuce).
Precipitation reactions may occur when cations and anions in aqueous solution combine to form an insoluble ionic solid (the precipitate). Such conditions, called saturation, occur when the concentrations of certain cations and anions in solution reach a maximum limit value (solubility). The concentrations of ions in equilibrium with the precipitate (i.e., solubility) can be calculated using a specific equilibrium constant called solubility product, which is tabulated for many chemical compounds and depends on temperature. Besides temperature, precipitation equilibria can be also influenced by other parameters such as pH and ionic strength (a parameter that considers the sum of the concentrations of all the ionic compounds in solution and their charge). Cations may form insoluble hydroxides at alkaline pH (by combining with OH − anions) or other insoluble precipitates by reacting with other anionic nutrients; thus, they must be carefully balanced and optimized to avoid losses from solution. In such cases, the values of pH and those of redox potential (Eh) must be continuously monitored or controlled. In this regard, pH values above 7 and positive Eh values may cause the precipitation of nutrients like iron (Fe), zinc (Zn), copper (Cu), nickel (Ni), and manganese (Mn II ) as insoluble (hydr)oxides. Precipitation of Fe III may occur already at pH well below the neutrality ( Takeno, 2005 ). At negative Eh values and acidic pH, e.g., in uncontrolled hydroponic systems under anoxic conditions, the same elements might also precipitate as insoluble sulfides, when sulfate is reduced to sulfide. At high pH values and high dissolved CO 2 concentrations, macronutrients like Ca and magnesium (Mg) can precipitate as carbonates. Precipitation of phosphates (mostly hydrogen phosphates) is another process to avoid in hydroponic solutions. This process, besides depleting phosphorus (P) from nutrient solution, may also reduce the solubility of other nutrients such as Ca, Mg, Fe, and Mn II . It is known that phosphate availability can be reduced at pH above 7 mostly due to precipitation with Ca. Different Ca-phosphate minerals can potentially form above this pH such as hydroxylapatite [Ca 5 (PO 4 ) 3 OH], amorphous tricalcium phosphate [Ca 3 (PO 4 ) 2 ], and Ca 4 H(PO 4 ) 3 ·3H 2 O ( Lee et al., 2017 ). Also sulfur (S) availability can be limited by precipitation with Ca, as Ca-sulfate minerals ( Packter, 1974 ). Silicon solubility is usually reduced at acidic pH, where SiO 2 precipitates may be produced ( Takeno, 2005 ). Precipitation/dissolution phenomena are often promoted by pH changes and therefore pH must be continuously controlled or buffered. Addition of nutrients in the form of salts to hydroponic solutions may lead to hydrolysis reactions, which may result in the acidification or alkalinization of the medium. Nitrogen (N) supply may also alter solution pH, if N is added only in the form of NO 3 − (alkalinization) or NH 4 + (acidification) ( Asher and Edwards, 1983 ). Yet, both N forms are usually added to hydroponic solutions. In general, saturation conditions for a certain nutrient could be reached if its concentration is increased due to water evaporation from the hydroponic system (owing to high temperatures or plant evapotranspiration). However, it has been recently observed that water losses by 20% (or even more) do not significantly influence precipitation equilibria ( Tomasi et al., 2015a ).
Strictly connected with precipitation are co-precipitation phenomena. These latter refer to the entrapment of an element (usually trace metals) within the structure of an insoluble compound (normally not containing that element) during its precipitation from solution. Co-precipitation may strongly reduce the solubility of nutrients added at trace concentrations like Cu, Zn, Mn II , and Ni, when insoluble species like Fe (hydr)oxides, Ca-carbonates, or Ca-phosphates are formed ( McBride, 1994 ). Co-precipitation is a process capable of strongly reducing the solubility of an element, well below that of the least soluble pure mineral phases of the element likely to form under environmental conditions ( Martínez and McBride, 2000 ).
Another important process to consider in hydroponic solutions is complexation, i.e., the formation of a chemical compound where a metal nutrient is bound by one or more neutral molecules or anions (ligands), either of organic or inorganic nature. The resulting complex can be a neutral compound, a cation, or an anion, depending on whether positive or negative charges prevail. Complexation reactions diminish the concentration of the free ions in the nutrient solution, changing elemental bioavailability. In general, the formation of complexes increases metal solubility even if very often, for some nutrients, they are less available for plant uptake than their free ions ( De Rijck and Schrevens, 1998a ). The addition, organic ligands such as EDTA, DTPA, EDDHA, citrate, etc. can increase the stability of certain elements in solution, especially Fe, Cu, Zn ( Lucena, 2003 ). However, as discussed later in this review, the different complexes may differently affect plant nutrient uptake and allocation (e.g., the case of Fe acquisition). In addition, some forms of soluble hydrogen carbonates and phosphates, as well as chlorides, can reduce the concentration of actual free metal cations in solution through complexation. The stability of complexes is another parameter that must be taken into account when preparing a nutrient solution: while on one side, efficient complexing agents can facilitate the solubilization of a nutrient in water, on the other side, strong complexes are usually more difficultly usable by plants ( Kraemer et al., 2006 ).
Considering all these aspects, it seems thus likely that the composition of a real hydroponic solution could reasonably be different from the planned one; being an altered availability of the nutrients in the growing medium a known risk for the quality of the vegetables ( Tomasi et al., 2015a ), the availability of tools for a realtime analysis of the nutrient solution composition is undoubtedly of particular usefulness and interest.
For a more accurate calculation of chemical equilibria in solution, ion activity should be considered instead of concentration. In fact, ion concentrations can be used only in calculations regarding ideal solutions, i.e., diluted solutions where no interactions among solutes occur. In real solutions, like in hydroponic solutions, interactions among solutes cannot be neglected and therefore ion activity should be used in calculations instead of concentrations. An ion activity is determined by multiplying the concentration of that ion in solution for an activity coefficient (≤1), which depends also on the concentration of all other ions in solution and their charge, i.e., on the ionic strength of the solution. Ion active concentration diminishes as the concentration of electrolytes in solution increases. For highly diluted (ideal) solutions, the activity coefficient for the chemical species in solution can be approximated to 1, and therefore ion concentrations are not influenced by phenomena such as ion-pair formation or ion conductivity reduction. But, when dealing with more concentrated solutions of strong electrolytes, like in hydroponic solutions, these phenomena become relevant and may reduce the ion active concentration. For example, for a sole KNO 3 solution at 6 mM concentration (as normally present in Hoagland nutrient solution), the activity coefficient for KNO 3 is about 0.92 and therefore its active concentration is 8% lower than the concentration actually added to solution. However, the activity coefficient of a single electrolyte depends on the ion strength of the whole solution and decreases with increasing concentration of all the electrolytes dissolved and of their charge. Usually, the concentrations of electrolytes in nutrient solutions for soilless cultivation are quite high, in the range 6.4–37.8 meq L −1 (corresponding to EC in the range 0.8–4.0 dS m −1 ) ( Savvas, 2003 ), and therefore the ion activity can be significantly reduced (e.g., the activity coefficient for KNO 3 is about 0.85, with an overall reduction of 15% active concentration).
All the abovementioned processes can be simulated and foreseen by using dedicated software to manage chemical equilibria calculations. Among these, Visual MINTEQ ( Gustafsson, 2013 ), MINEQL+ (Environmental Research Software, USA), CHEMEQL ( Müller, 2015 ), CHEAQS ( Verweij, 2017 ) are some of the most used both in water and soil chemistry. All the mathematical models employed by these software tools are based on thermodynamic data and usually kinetic parameters are not taken into account. However, it is known that kinetic constraints can prevent a process from occurring or limit its representativeness ( Terzano et al., 2015 ). For instance, with respect to the precipitation processes, oversaturation or under-saturation phenomena can change nutrient solubility, respectively, above or below the concentration allowed by thermodynamics.
In a context of a more sustainable agriculture, a specific comment must be dedicated to the exhausted hydroponic solutions at the end of a productive cycle. In this regard, it is clear that they represent an interesting resource in terms of water and fertilizer savings, which is becoming increasingly relevant especially in those countries where there is shortage of rain or good-quality water and farmers cannot afford the expenses to buy large amounts of fertilizers. Moreover, recycling exhausted solutions may also represent an efficient strategy to prevent groundwater and environmental pollution, especially from intensive agricultural productions. However, the main problem with the reuse of exhausted nutrient solutions is the shortage of some key macro and micronutrients ( da Silva Cuba Carvalho et al., 2018 ) and their increased salinity ( Grattan and Grieve, 1998 ; Pardossi et al., 2005 ; Bar-Yosef, 2008 ) causing, in turn, problems for crops ( Carmassi et al., 2005 ; Parida and Das, 2005 ) even if to different extents from one plant species to another ( Bar-Yosef, 2008 ). Thus, a research challenge in this context is surely to develop management practices/tools that reduce salinity in recycled solutions and/or minimize the physiological impact of salinity on plants ( Neocleous et al., 2017 ). In addition, considering the composition of a typical Hoagland solution and according to chemical equilibrium models (Visual Minteq 2.61), at pH 5.5, according to thermodynamic data, few other nutrients could easily reach oversaturating conditions if certain ions accumulate in exhaust Hoagland solutions, mostly phosphates (precipitating as Ca, Mg, or Fe phosphates) and molybdate (as Ca molybdate). Also, this aspect needs a careful monitoring when a reuse of the hydroponic solution for another cycle is planned.
Salinity increase could be contrasted by treating the recycled water with appropriate osmotic systems, including forward and reverse osmosis. In the last years, particular attention has been paid to forward osmosis (FO) technologies, also with the purpose to reuse wastewater for fertigation purposes ( Van der Bruggen and Luis, 2015 ). In this sense, Phuntsho et al. (2012) proposed the use of concentrated fertilizer solutions as draw solutions in FO systems. This application maybe of interest for hydroponic cultures particularly when the feed water is of a low quality, like an exhausted nutrient solution or wastewater. The concentrated fertilizer solution is used to withdraw pure water from this source (see the scheme in Figure 1 ). In this way, water is recycled and is also enriched in those nutrients that have been lost by plant uptake or other chemical processes (e.g., precipitation, complexation, sorption). This technology, called fertilizer drawn forward osmosis (FDFO), has been recently applied to grow hydroponic lettuce using a commercial nutrient solution ( Chekli et al., 2017 ). In a pilot-scale study, these authors demonstrated that the FDFO process is able to produce the required nutrient concentration and final water quality (i.e., pH and conductivity) suitable for hydroponic applications and the hydroponic lettuce showed similar growth patterns as the control without any sign of nutrient deficiency.
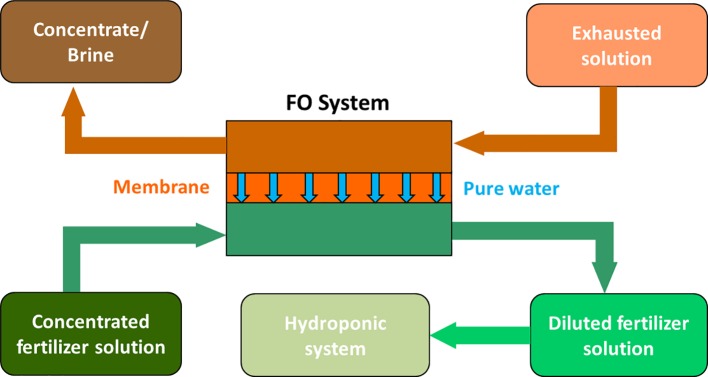
Scheme of a fertilizer drawn forward osmosis desalination process for nutrient solution preparation to be used in hydroponic cultivation systems (modified from Phuntsho et al., 2012 ).
Another relevant issue when dealing with closed-loop hydroponic cropping systems is the accumulation of potentially toxic organic compounds released by the roots of cultivated plants. This phenomenon is known as allelopathy/autotoxicity ( Kitazawa et al., 2005 ; Asaduzzaman and Asao, 2012 ) and occurs when a plant species releases chemical substances which inhibit or delay germination and/or growth of the same plant species ( Singh et al., 1999 ; Mondal et al., 2015 ). Among these substances, benzoic acids were mainly found to inhibit the growth and yield of several crops ( Hosseinzadeh et al., 2019 ). To overcome this issue, a number of treatment techniques have been proposed for root exudates degradation or removal, either physical (e.g., activated carbon adsorption, ion exchange resins, membrane filtration, sand filtration); chemical (e.g., TiO 2 photocatalysis, UV treatments, ozonation, electrodegradation); or biological (e.g., use of degrading microbial strains) ( Asaduzzaman et al., 2012 ; Mondal et al., 2013 ; Hosseinzadeh et al., 2017 ; Talukder et al., 2018 , 2019 ). All these treatments may have advantages and drawbacks as reviewed by Hosseinzadeh et al. (2017) . In general, to be effective, the treatment technique applied should be able to remove root exudates without interfering with the inorganic mineral nutrients in solution. One of the main problems in this sense is the degradation/adsorption of Fe chelates ( Ehret et al., 2001 ).
Nutrient Chemical Forms and Uptake Processes
With respect to the chemical forms of a nutrient, N represents one of the most explicative examples. In fact, it is well demonstrated that plants can use a wide variety of N forms, ranging from the inorganic, namely NH 4 + and NO 3 − , to the organic ones, as for instance urea and amino acids ( Nacry et al., 2013 ). Due to environmental conditions and plant species, the relative contribution of each N form to plant N supply can be quite variable ( Haynes and Goh, 1978 ; Schimel and Chapin, 1996 ; Marschner, 2012 ). From a metabolic point of view, the assimilation of NH 4 + is less expensive as compared to NO 3 − , NH 4 + being directly usable to generate glutamine ( Marschner, 2012 ). However, a pure NH 4 + nutrition has been shown to cause the development of toxicity symptoms in many herbaceous plants, as well as to inhibit NO 3 − uptake ( Kronzucker et al., 1999 ). Therefore, a balanced N diet (NO 3 − and NH 4 + ) is clearly beneficial for several plant species as compared to that based exclusively on NO 3 − ( Forde and Clarkson, 1999 ). Accordingly, it has been observed in tomato plants that the root growth was optimal when NO 3 − and NH 4 + were supplied in a 3:1 ratio; on the contrary, when NH 4 + concentration was too high, a strong inhibition in the root development was observed ( Bloom et al., 1993 ). A more recent study in watermelon plants showed that with decreasing NO 3 − /NH 4 + ratio, the leaf number, leaf area, shoot height, net photosynthesis, biomass, and root growth significantly decreased, as well as the concentration of several macronutrients ( Na et al., 2014 ). Nevertheless, it has also been observed that the optimal NO 3 − / NH 4 + ratio to be applied for cultivation can be dependent on both the plant species and the environmental conditions (e.g., salinity) ( Errebhi and Wilcox, 1990 ). For instance, in canola plants grown in salinity conditions, a NO 3 − /NH 4 + ratio of 1:1 is suitable to minimize the negative effects produced by the abiotic stress, thus maximizing the yield and quality ( Bybordi et al., 2012 ). Overall, these results clearly indicate that not only the forms of N but also the concurrently presence of more than one form and in specific ratios among these forms are decisive for specific productive objectives.
Also in the case of Fe nutrition, the nature/forms of the Fe sources utilized can play an important role not only at the level of its uptake but also in the Fe allocation within the plant. In natural environments (soil grown cultures), Fe is present in inorganic mineral forms (e.g., goethite, hematite, and ferrihydrite), which are poorly bioavailable ( Colombo et al., 2013 ; Mimmo et al., 2014 ). Therefore, plants need to rely on a series of natural ligands for the mobilization of the micronutrient from the insoluble sources and to guarantee appropriate levels of Fe availability ( Cesco et al., 2000 ; Tomasi et al., 2013 ). However, often this pool of Fe sources cannot satisfactorily sustain an equilibrate growth of plants triggering the onset of Fe deficiency symptoms. The Fe supply (in terms of quantities and usable forms) is an issue also for soilless crops. Several pieces of research have compared in hydroponic solutions the efficiency of different organic ligands complexed with Fe as micronutrient source for plants ( Pinton et al., 1999 ; Tomasi et al., 2009 , 2013 , 2014 ). These reports clearly demonstrated that Fe complexed by water-extractable humic substance (WEHS) fractions can be used with higher efficiency than other natural or synthetic sources ( Pinton et al., 1999 ; Tomasi et al., 2009 ; Zanin et al., 2019 ), favoring a faster recovery from the deficiency symptoms ( Tomasi et al., 2014 ). The effect appears to be not exclusively ascribable to phenomena occurring at the root level ( Tomasi et al., 2013 ; Zamboni et al., 2016 ), but also to those happening in the shoot/leaves ( Tomasi et al., 2009 , 2014 ). Therefore, from these experimental evidences, it is clear that different Fe sources can be used with different efficiencies, which are the result of a complex response, including biochemical-physiological-molecular aspects, involving the whole plant. However, despite all this knowledge, little still exists in the literature in relation to the application of these findings in the soilless cultures at the production scale. This aspect is even more critical if the increasing demand for Fe-enriched vegetable products is considered.
When nutrient ions are analyzed in hydroponic solutions, one aspect to consider is also the nature of the counter-ion forming the ion pair in solution. In fact, it is well demonstrated that anion which is taken up relatively slowly can also reduce the uptake speed of its counter-ion, as observed for SO 4 2− on potassium (K + ) uptake ( Marschner, 2012 ). Also, the root capability to extrude protons in the external medium, generating the electrochemical gradient across the plasma membrane at the base of the active nutrient uptake, is consistently influenced by this phenomenon ( Pinton et al., 1997 ). With respect to the hydroponic solutions of soilless cultures, the simultaneous presence of different cations and anions (also at different concentrations) unfortunately makes the phenomenon even more complex and difficult to understand and to manage. However, although the impact of this phenomenon on the overall process of radical nutrient acquisition has long been clear, up to now, there are no specific studies applied to the hydroponic system.
Nutrient Interactions
It is well known that, at the level of the nutrient acquisition mechanisms of roots, competitive or antagonistic phenomena among elements can occur ( Marschner, 2012 ), leading, in some contexts, also to relevant nutritional disorders affecting the whole plant. A clear example is represented by the interaction between NH 4 + and K + . The acquisition of NH 4 + involves an active transport which is very specific for the cation ( von Wirén and Merrick, 2004 ). Differently, K + acquisition is described as a biphasic transmembrane transport of high and low affinity transporters ( Gupta et al., 2008 ; Zhang et al., 2012 ; Shin, 2014 ). However, the selectivity of these K + transporters is definitely lower than that of others, including the NH 4 + ones ( Gierth and Mäser, 2007 ). In this regard, it is interesting to note that an inhibition of K + uptake by NH 4 + has been already described, whereas the vice versa does not happen ( Mengel et al., 1976 ; Rufty et al., 1982 ; Shaviv et al., 1987 ). This phenomenon has been ascribed to the capability of K + transporters to catalyze also the transmembrane transport also of NH 4 + ( ten Hoopen et al., 2010 ). Certainly, the similarity of the two cations (NH 4 + and K + ) in terms of valence and ion diameter is, at least in part, at the base of the phenomenon. From the agronomical point of view, this aspect could be crucial for NH 4 + -fed plants when exposed to a sub-optimal/unbalanced availability of K + because the competition could induce/exacerbate K + deficiency ( Zhang et al., 2010 ). This aspect could be of particular relevance when the additional application of NH 4 + is of pivotal role to achieve specific qualitative objectives of the edible fruits ( Valentinuzzi et al., 2018 ).
It is also worth mentioning that the interactions K + /Na + and Cl − /NO 3 − could represent a limiting factor for soilless cultivation of crop plants, especially in semiarid environment characterized by saline water. It is well known that NaCl interferes with the uptake processes of both K + and NO 3 − , since K + is sensitive to sodium (Na + ) in the external environment, while the uptake of NO 3 − is inhibited by chloride (Cl − ) ( Silberbush and Ben-Asher, 1989 ). This phenomenon could be even more pronounced in hydroponic solutions particularly when used for more than one cycle (closed system) (i.e., exhausted hydroponic solutions).
A further example of competitive interaction between nutrients is that occurring among Ca 2+ , Mg 2+ , and K + ( Marschner, 2012 ), although observed up to now only in soil-grown crops. It is well known that a high availability of K + and Ca 2+ , most often after an unbalanced fertilization practice, can induce Mg 2+ deficiency in crop plants. At the moment, the mechanisms underlying such interactions have not been clarified. However, as suggested by Schimansky (1981) , the excessive availability of the other two cations (K + and Ca 2+ ) could reasonably inhibit Mg 2+ uptake by roots. In this context, and in particular with respect to hydroponic cultures, monitoring the ratio between these three cations in the solutions is without any doubt advisable in order to avoid K + /Ca 2+ -induced Mg 2+ deficiency, particularly when closed systems are considered.
Also Fe acquisition mechanism offers the possibility to describe several examples of interactions among elements/nutrients. In dicots and non-graminaceous monocots, Fe is taken up through the IRT1 transporter ( Connolly et al., 2003 ), which was shown to transport also other divalent cations such as Mn 2+ , Zn 2+ , and Cu 2+ ( Korshunova et al., 1999 ). In sub-optimal availability of Fe ( Zamboni et al., 2016 ), a marked accumulation of these elements in plant tissue has been observed, with also a different spatial allocation of each element ( Tomasi et al., 2014 ). On the other hand, a rather worrying aspect affecting the quality of the edible plant tissue is that the IRT1 transporter can also mediate the uptake of toxic elements like cadmium (Cd) ( Astolfi et al., 2012 , 2014 ; He et al., 2017 ). This last evidence clearly highlights how crucial is the quality of the nutrient sources adopted as well as that of the water used to prepare the hydroponic solution (in terms of trace elements), to avoid unexpected contamination of crops, the worsening of the edible parts’ quality, and threatening the food safety ( Zhao et al., 2010 ).
Another interesting example of nutrient interaction has been described by Prosser et al. (2001) using S-starved spinach plants ( Prosser et al., 2001 ). In this experience, the authors recorded as a consequence of S starvation a consistent accumulation of NO 3 − in leaves of plants, one of the greatest issues concerning hydroponic productions ( Santamaria, 2006 ). It is interesting to note that this phenomenon seems to be not specific for S, since it has also been observed for Fe-starved cucumber plants ( Nikolic et al., 2007 ). In both these cases, although the uptake of NO 3 − was hampered by the two nutrient shortages (S or Fe), the effect on the assimilation process seems to play a dominant role in determining the NO 3 − accumulation at the leaf level. However, such nutrient interactions (S vs. N and Fe vs. N) seem to be pretty complex and not so easy to simply explain in terms of competition/antagonism phenomena for their transmembrane transporters. In fact, it has also been demonstrated that Fe uptake and the mechanism underlying the process (i.e., the Fe 3+ reduction step mediated by the plasma membrane reductase FRO) are strongly influenced by the availability of N ( Nikolic et al., 2007 ). Interestingly, Zuchi et al. (2009) have demonstrated that the same Fe acquisition mechanism is severely affected also by S availability. Although the overall impact in terms of nutrient acquisition appears to be essentially the same, the components of the transport machinery seem to be differentially affected by the starvation of the two nutrients; in fact, while FRO is inhibited by both N and S starvation, the transport of Fe 2+ through IRT1 seems to be hindered only by limited availability of S. These data highlight that the uptake of each single element (e.g., N, S, or Fe) is not exclusively dependent on its availability in the hydroponic solution but also on the presence (and availability levels) of other elements. Thus, it appears evident that an optimized and well-balanced supply of nutrients is a prerequisite for an efficient use of the resources by hydroponically grown vegetables, not only to ensure a high yield but also to guarantee the quality of the edible tissues. Interestingly, it has been demonstrated that a higher Fe accumulation in plant tissues (Fe fortification) can be reached by means of a sulfate over-fertilization ( Zuchi et al., 2012 ).
In the context of nutrient interactions, it is interesting to highlight that these phenomena have been observed also in the case of biofortification programs like with Se. Generally, the biofortification of vegetables is obtained by the application of Se, in the form of selenate, in the nutrient solution, this form being the most available for plants ( Schiavon and Pilon-Smits, 2017 ). Due to the similarities in the chemical and physical features ( Shibagaki et al., 2002 ; El Kassis et al., 2007 ), the acquisition of selenate follows the same pathway exploited by SO 4 2− , based on the sulfate transporters SULTR ( Malagoli et al., 2015 ). For this reason, it is clear that, competing for the same transporter, a sort of antagonism between sulfate and selenate can occur. As demonstrated by ( Hopper and Parker, 1999 ), a high availability of sulfate might prevent the acquisition of selenate, thus vanishing the potential biofortification of the edible parts.
Differently, the experiences aimed at Si biofortification in agricultural crops, both ready to eat and to be transformed ( Gottardi et al., 2012 ; Montesano et al., 2016 ; D’Imperio et al., 2016a ), did not reveal interaction phenomena with other nutrients, at least with negative outcome on the plants’ whole nutrient balance. Several authors have reported a mitigation effect of Si in P-starved crop plants in soilless cultivation systems ( Ma and Takahashi, 1989 ; Ma, 2004 ); similarly, it has been observed that the supplementation of Si in the nutrient solution of Fe-deficient dicots can alleviate Fe chlorosis ( Gonzalo et al., 2013 ; Pavlovic et al., 2013 ). Indeed, Gottardi et al. (2012) showed that the Fe reduction and uptake rate at root level were up-regulated by Si supplementation, although Fe translocation to leaves was not influenced. On the other hand, the effect of Si on Fe uptake/allocation in dicots is still debated ( Liang et al., 2015 ). In addition, the Si fertilization might not be only important for Si biofortification approaches, but also to improve plant fitness. The application of silicate fertilizers to paddy fields worldwide has resulted in an increased yield in rice production, with a higher photosynthesis rate and a better light interception due to an improved leaf blade position ( Tamai and Ma, 2008 ). These last aspects, even if observed in soil-grown crops, should also be taken into consideration for soilless cultures for the undoubted advantages that they could guarantee.
Perspectives
The issues described earlier clearly highlight how unexpected physicochemical phenomena happening in the hydroponic solution can easily modify its composition as well as a series of nutrient interactions can seriously alter the efficiency of the nutrient acquisition process of crops. These phenomena considered together are able, unfortunately, to considerably affect the production of the soilless system based on hydroponics, both from a quantitative and qualitative point of view. For this reason, the availability of new forms of nutrients (nanoparticles, section “Nanoparticles”) and/or of bioeffectors able to enhance the functionality of the root nutrient acquisition mechanisms (PGPRs, section “Use of Plant Growth-Promoting Rhizobacteria in Hydroponic Solutions”) may be of particular relevance. Similarly, the possibility of exploiting tools to monitor the composition of a nutrient solution in realtime (sensors, section “ Realtime Monitoring of Hydroponic Solutions via Sensors”) and to analyze the data (interpretation algorithms, section “Interpretation Algorithms and Smart Agriculture”) for a prompt correction may clearly facilitate a more efficient use of the hydroponic solutions.
Nanoparticles
In the agricultural context, the use of nanoparticles (NPs) is mainly aimed at reducing nutrient losses in the environment as well as at increasing yields through an optimal management of nutrients and water. In fact, thanks to their high specific surface and relevant reactivity (having a particle size lower than 100 nm), nanoparticles might supply the plant with more soluble and available forms of nutrients limiting precipitation and insolubilization processes often described for several fertilizers (e.g., phosphate ones) ( Liu and Lal, 2015 ). For this reason, in comparison to the traditional fertilizers, nanoparticles are considered much more efficient carriers of nutrients for plants ( Nair et al., 2010 ; Campos et al., 2014 ; Roosta et al., 2017 ). These advantageous aspects are valid not only for the soil system but even more for the soilless systems (considering also what described for chemical equilibria in hydroponic solution, section “Chemical Management of Nutrient Availability in the Hydroponic Solution”). Therefore, nanoparticles surely represent a promising tool in general, specifically for the soilless growing systems. The use of nanoparticles in soilless cultivation has also been evaluated in the ability to control potential pathogens during cultivation through the use of NP of silver (Ag), Cu, Si, titanium (Ti), and Zn improving the plant defense ( Amooaghaie, 2011 ). Elmer and White (2016) tested the use of metallic oxide nanoparticle to enhance tomatoes’ and eggplants’ growth. Their results showed that NP of CuO increased fresh weights by 64%, reducing the Verticillium wilt fungus by 69%, having 32% more Cu in the roots. Within metallic NP, growth enhancement was recorded also for hydroponic spinach treated with iron oxide (Fe 2 O 3 ) NP ( Jeyasubramanian et al., 2016 ). The mechanism of Fe uptake by the spinach plant from the Fe 2 O 3 NP can be explained as follows: in general, the uptake of Fe 3+ is found to be pH sensitive and naturally Fe 3+ is insoluble which will be slowly converted into Fe 2+ under acidic environment. This determined an increase in root and shoot length, biomass, and Fe content in a dose-dependent manner. NP can also be used to recover waste nutrient solution from soilless cultivation systems. An example is given by the photocatalytic treatment of waste nutrient solution coming from tomato cultivation in rice hull substrate ( Miyama et al., 2009 ). In this experiment, the substrate was treated with TiO 2 -coated porous alumina filter, that when irradiated with ultraviolet light, exhibits a strong oxidation effect, decomposing organic compounds. By this process, the phytotoxic compounds could be decomposed and detoxified and the nutrient solution recycled. Moreover, it was observed that tomato growth in the photocatalytically treated system was significantly higher than control in six experiments over 3 years and yields were comparable to those in a currently used open cultivation system using stonewool substrate. In addition to these interesting aspects, it has been clearly ascertained that nanoparticles are able also to affect key processes of plants including germination, seedling vigor, root growth, photosynthesis, and even flowering ( Lin and Xing, 2007 ; Tripathi et al., 2016 ). Moreover, a protective action of nanoparticles against oxidative stress in plants has been recently demonstrated; the phenomenon was ascribed to the capability of these particles to mimic the role played by antioxidant enzymes (e.g., superoxide dismutase, catalase, and peroxidase) ( Burman et al., 2013 ). Khan et al., (2017) have shown that other abiotic stresses (such as temperature, salinity, drought stress) can be alleviated by nanoparticles as well as drought-stress resistance in plants can be enhanced by applying nanoparticles ( Tripathi et al., 2017 ). In particular, the application of NPs of analcite ( Zaimenko et al., 2014 ) significantly alleviated the drought stress in wheat and corn by increasing the photosynthetic pigments and the accumulation of protective antioxidants in these plant species. Recently, nanomaterials are used as an important tool for increasing the growth and yield of crops under salinity condition ( Khan et al., 2017 ). The application of nano-Si for example significantly reduced salt stress, increasing seed germination and antioxidative enzyme activity, photosynthetic rate, and leaf water content ( Haghighi and Pessarakli, 2013 ; Qados, 2015 ). In addition, these mitigating effects have been also noticed in other plant species such Ocimum basilicum ( Kalteh et al., 2018 ) Cucurbita pepo ( Siddiqui et al., 2014 ), and Vicia faba ( Qados and Moftah, 2014 ).
With respect to temperature stress, this can either be due to too high or too low temperature. Loss of fluidity of membranes and leakage of solutes are typical symptoms of cold stress. In this regard, nanomaterials such as TiO 2 may help in alleviating the dangerous effects of cold stress by limiting the membrane damages and electrolyte leakage ( Mohammadi et al., 2013 ). On the other hand, heat stress accelerates the overproduction of reactive oxygen species and increases oxidative stress leading to disintegration of membrane lipids, leakage of electrolytes, and denaturation of biomolecules ( Karuppanapandian et al., 2011 ). It has been shown that, among nanomaterials, low concentration of selenium (Se) can alleviate the effects of heat stress for its antioxidative properties ( Haghighi et al., 2014 ).
However, despite these pieces of evidence, there are still several open questions about the molecular mechanisms underlying the aforementioned phenomena. In addition, the phytotoxic effects of nanoparticles (presumably via an enhanced production of reactive oxygen species) described by Khan et al. (2017) make the issue even more complex and less clear. Moreover, little is yet known about their capacity to enter, through the edible tissues, in the food chain and, even more critical, about what effects they may have on human health and the environment. For these reasons, right now, the exploitation at the field scale of these particles appears to be quite premature; in fact, a cautionary principle reasonably prevails. In summary, despite nanoparticles representing a promising tool, further studies are needed to evaluate the impacts on crop growth, quality, and safety ( Gardea-Torresdey et al., 2014 ) before their massive use in the agricultural production system.
Use of Plant Growth-Promoting Rhizobacteria in Hydroponic Solutions
As widely addressed in literature, the molecular machinery used by plants for the acquisition of mineral elements is characterized by an extreme plasticity in order to guarantee the adaptability to the nutrient fluctuations in the growth medium. Such variations, particularly in the bioavailable nutrient fraction, can be ascribed to several factors, as for instance the concentration and type of nutrient source, the pH, and the redox potential ( Tomasi et al., 2009 ; Marschner, 2012 ; Mimmo et al., 2014 ). In addition, a recent body of evidence has drawn the attention to the role played by the plant growth-promoting rhizobacteria (PGPRs) in contributing to the mineral nutrition of plants ( Pii et al., 2015 ). The different mechanisms brought about by PGPRs aimed at increasing the bioavailability of mineral nutrients in the rhizosphere (i.e., atmospheric N 2 fixation, P solubilization, siderophores production for Fe 3+ chelation) have been extensively investigated and reviewed ( Lugtenberg and Kamilova, 2009 ; Glick, 2012 ; Pii et al., 2015 , 2019 ; Alegria Terrazas et al., 2016 ). Nevertheless, recent pieces of research have highlighted that the PGPRs can themselves induce alteration in the functionality of the molecular machinery devoted to nutrient acquisition ( Pii et al., 2015 ). In this sense, PGPRs were shown to alter the release of protons from wheat roots and other model plants, thus supporting the hypothesis that they could have a direct effect on plasma membrane (PM) H + -ATPases ( Bashan et al., 1989 ; Bashan, 1990 ; Bertrand et al., 2000 ; Canellas et al., 2002 , 2013 ). Considering that the H + electrochemical gradient at level of the PM is necessary for the absorption of several mineral nutrients, like H 2 PO 4 − , SO 4 2− , and NO 3 − ( White, 2003 ), the increased PGPR-induced H + release could indeed be reflected in a higher ability of plants to take up nutrients. Similarly, the bacteria Achromobacter were shown to induce an increase in the concentration of NO 3 − in plant tissues, most likely for its action on the constitutive high-affinity transport system (cHATS) for NO 3 − ( Bertrand et al., 2000 ; Nacry et al., 2013 ). Furthermore, the influence of PGPRs was also demonstrated on the molecular mechanisms underpinning the acquisition of Fe (Fe 3+ reduction – FRO, Fe 2+ transport – IRT1, and rhizosphere acidification – PM H + -ATPase) in dicots plants. The fungus Trichoderma asperellum could stimulate Fe uptake in Fe-sufficient cucumber and Lupinus albus by enhancing the activity of the root Fe-chelate reductase ( de Santiago et al., 2013 ; Zhao et al., 2014 ), while Bacillus subtilis GB03 could induce the expression of genes coding Fe-chelate reductase and the PM H + -ATPase in Arabidopsis thaliana ( Zhang et al., 2009 ). Recently, Pii et al. (2016) demonstrated that the PGPR Azospirillum brasilense can affect the Fe acquisition machinery in cucumber plants independently from the Fe nutritional status, thus suggesting that the different actors (Fe-chelate reductase, Fe 2+ transporter, PM H + -ATPase) of the mechanism underlying Fe acquisition in roots can undergo a different regulation following PGPR inoculation. The majority of these studies have been carried out in soil conditions, while very little is known about the real performance of PGPRs in hydroponic systems, where their actions also depend on the ability of thriving and proliferating in specific environments, as well as, on colonizing the plant roots ( Lee and Lee, 2015 ). However, several bacterial strains have been already successfully tested in hydroponically grown fruits and vegetables, obtaining positive effects on the yield and the quality of the agricultural products. The PGPRs belonging to Bacillus spp. have been tested for their effect on the growth and productivity of both tomato and pepper plants cultivated in hydroponic conditions ( García et al., 2004 ; Gül et al., 2008 ). In particular, B. licheniformis was shown to significantly enhance the height of plants and the leaf area in both pepper and tomato plants, the effect being species-dependent ( García et al., 2004 ). Nevertheless, the inoculation induced an increase in the number and in the diameter of tomato fruits ( García et al., 2004 ). Similarly, the inoculation of tomato plants with the commercially available PGPR B. amyloliquefaciens (FZB24 and FZB42) resulted in an increase in the fruit yield of about 8–9% ( Gül et al., 2008 ). Further studies have demonstrated that the application of specific PGPRs, such as B. sphaericus UPMB10 and A. brasilense Sp7, which are able to carry out the biological nitrogen (N 2 ) fixation, might contribute to reducing the external input of nitrogen sources in the hydroponic solution used for the soilless cultivation of banana plants, still guaranteeing an adequate plant production ( Baset Mia et al., 2010 ). Interestingly, A. brasilense was also demonstrated to increase the size of strawberries delivered by inoculated plants, to enhance the nutraceutical qualities (i.e., flavonoids, flavonols, and micronutrients concentration) as well as the sweetness index of fruits ( Pii et al., 2018 ). Nonetheless, hydroponically grown plants in indoor systems might be threatened by pathogen attack; therefore, disinfection practices, either physical (e.g., ultraviolet light, gamma radiations) or chemical (e.g., the use of using carbendazim, hymexazol, imidazole, prochloraz triazole), could be required ( Lee and Lee, 2015 ). Indeed, these control methods can result also in a decrease of PGPR population in the hydroponic systems, thus representing a limitation to the application of beneficial microorganisms to the hydroponic cultivation systems ( Hibar et al., 2006 ). On the other hand, the use of PGPRs that feature also biocontrol traits might represent a valuable alternative to the abovementioned disinfection procedures.
Overall, these observations prefigure a very interesting scenario, in which the potential application of beneficial microorganisms (PGPRs) in the hydroponic cultivation of plants could lead to a further improvement in the productivity and in the nutraceutical properties of crops ( Lee and Lee, 2015 ). Indeed, this practice would also impact on the sustainability of the agricultural systems, allowing a rationalization in the use of water resources and in the external inputs of agrochemicals (e.g., fertilizers and pesticides).
Realtime Monitoring of Hydroponic Solutions via Sensors
The hydroponic cultivation of small or medium-size fruiting crops, nowadays gaining ever more importance for the high productivity per area of cultivation ( Bradley and Marulanda, 2001 ), often is based on closed-loop systems on field scale. In this case, the hydroponic solutions are often utilized for more than one single culture cycle; therefore, they need to feature high concentrations of mineral elements in order to guarantee an adequate nutrient supply for plants’ growth in the repeated cycles ( Tomasi et al., 2015a ). However, the selective removal of nutrients due to plant growth, as well as the evapotranspiration process, could in any case change the concentration of nutrients in the hydroponic solution as well as the accumulation of undesired counter-ions (i.e., Na + , Cl − ), and have also an impact on the electrical conductivity (EC) of the solution itself ( Wild et al., 1987 ; Zekki et al., 2019 ). Since such alterations in the qualitative and quantitative composition of the substrate solution can adversely impact on crop yield and quality, the need of timely tuning the nutrient solutions is of paramount importance to guarantee an adequate production ( Cho et al., 2017 ). A possible tool for the on-line monitoring of hydroponic solutions can be represented by the use of ion-selective electrodes (ISEs) that are also applied for the assessment of drinking water quality ( Melzer et al., 2016 ). Several authors have already applied this technology to measure the multiple components of a nutrient solution in order to ensure the optimal composition required for plant growth ( Chen et al., 2011 ; Cho et al., 2017 ). However, at present, many studies have been carried out by measuring one or few plant macronutrients at a time ( Bailey et al., 1988 ; Bamsey et al., 2012 ; Kim et al., 2013 ; Rius-Ruiz et al., 2014 ; Vardar et al., 2015 ), and this prevented obtaining a complete and realistic picture of the elements’ availability in the nutrient solution. In addition, to the best of our knowledge, selective electrodes for the determination of plant micronutrients, as for instance Fe, Cu, and Zn, have not been developed and/or applied yet. In addition, the use of ISEs as on-line monitor systems may present issues related to the signal drift and the reduction in sensitivity over time, due to a continuous exposure to the nutrient solutions, without appropriate calibration procedures ( Gutiérrez et al., 2007 , 2008 ; Bamsey et al., 2012 ; Kim et al., 2013 ). The development of a new generation of ISEs through the overcoming of the aforementioned technical limitations (i.e., simultaneous macro and microelements measurements, longer signal stability) might represent a useful tool for the on-line and realtime monitoring of nutrient solutions, with the aim of satisfying the nutritional requirements of crop plants for optimal growth.
Interpretation Algorithms and Smart Agriculture
The sensors and the other new technologies described in section “ Realtime Monitoring of Hydroponic Solutions via Sensors” create massive flows of data that should then be analyzed in order to be adequately exploited. Machine learning algorithms (such as neural networks and genetic algorithms) might be applied for self-calibrating and managing the parameters of hydroponic solutions based on sensors data ( Morimoto and Hashimoto, 1996 ; Suhardiyanto et al., 2009 ). The constant control of the composition and concentrations could in fact allow the recirculation and reuse of nutrient solutions within closed growing systems, thus reducing the economic costs and minimizing the environmental impact of soilless cultivation systems ( Jung et al., 2015 ). Similarly, advanced Big Data analytics and simulation techniques might allow to forecast the quality and quantity of vegetable or fruit production under various conditions – for instance by creating a realtime “digital twin” of the real/physical hydroponic system – and in turn to determine the optimal parameters, such as the composition and concentration of the hydroponic nutrient solution; the temperature, humidity, and CO 2 levels (in case of greenhouses); and the lighting (in case of greenhouses with artificial light). Finally, Internet of Things and cloud computing might be employed to share the data among different farmers and make the data analysis more efficient and effective.
The use of sensors and data analysis tools is fully included in the concept of precision agriculture (PA) – or smart agriculture (SA) as it has been recently labeled – a new paradigm based on the use of information and communication technologies in the cyber-physical farm management cycle. More formally, PA has been defined as a “management strategy that uses information & communication technologies (ICT) to collect data from multiple sources in view of their later use in decisions concerning production activities” ( National Research Council, 1997 ). Originally, this definition was intended to refer to field processes, being mainly focused on the highly automated site-specific approaches, aiming at overcoming the management limits imposed by the relevant spatial variability in field properties. Later, it was extended to many other types of farming systems, such as livestock, viticulture, and orchards. SA is based on the so-called Knowledge Management approach (also renamed as Knowledge Management 4.0 or KM4.0; Calcante and Mazzetto, 2014 ; Mazzetto et al., 2017 ; Meško et al., 2017 ; Neumann, 2018 ), which includes the following aspects: (1) ensuring an approach highly oriented to the Internet of Things (IoT) knowledge processes; (2) managing and treating Big Data acquired directly from things (= elements of processes and products) and customers (people acting in the system); (3) sharing information between people or things without any limitation; (4) storing all data and information directly in clouds through Internet of Services (IoS); (5) ensuring that all contents are always available online, also to implement any realtime automation process; (6) providing information sharing (C2C, C2M) via wireless solutions (hyper-connectivity); (7) fostering of predictive analysis in the main maintenance and control tasks.
Hydroponic systems represent an ideal application context for SA ( Dinesh et al., 2018 ) since they are closer to the industrial context: production processes are carried out in contained and more controllable spaces (greenhouses or tunnels); they are more repeatable (lighting, climatic parameters, and nutrient supply can be controlled); and they can be more easily automated. In the hydroponic practices, there are already some technological examples moving just toward such a direction. Fujitsu, ORIX Corporation, and Masuda Seed launched in 2016 the Iwata Smart Agriculture Project, which aims at collecting a wide set of data concerning the optimal parameters (temperature, humidity, CO 2 levels, and nutrient concentration of hydroponic solutions) for hydroponic production of various seed varieties and sharing them through Internet. The (Big) Data are collected by Fujitsu in a plant factory located in the city of Iwata (Japan) and consisting of several sensorized and fully controlled greenhouses and then analyzed through the Microsoft’s Azure cloud, also relying on machine learning algorithms. They are then made available to farmers (both professional and amateur farmers) through the IoT and cloud computing platform Akisai Food and Agriculture. Ray (2017) provides an overview of various commercial IoT-based agriculture sensor systems, some of which are suitable for hydroponics and aquaponics farming (e.g., Bitponics, Open garden, and Niwa). Other studies ( De Silva and De Silva, 2016 ; Yolanda et al., 2016 ; Charumathi et al., 2017 ) propose instead “new” IoT architectures for hydroponics and aquaponics farming, based on sensors, data loggers, actuators, and software tools (e.g., Arduino).
Conclusions
This overview of some issues affecting nutrient solutions in soilless cultivation systems clearly highlights the main working topics in which the research world is involved in the field. The huge potential offered by this cultivation approach is indisputable and ranges from productive and qualitative advantages to environmental benefits due to higher efficiency in the use of water and nutritional resources. Currently, there are well-studied and tested research areas whose results are commonly exploited in the soilless cultivation such as the NO 3 − management or the crop quality increase by managing the electric conductivity of the solution. On the other side, besides these positive aspects, there are others, more difficult to manage, related to the interactions among nutrients in their acquisition processes and the nutrient dynamics (bio-geochemical cycles) in the hydroponic solution. These aspects represent a significant discriminant for the soilless management and, in some cases, may limit its diffusion, since the growers must possess specific knowledge and detailed skills to cope with and specifically per each crop species. It is interesting to note that parallel to the research areas dealing with these aspects, there are others newly emerging, technologically advanced but still not widely studied, even if they seem to provide promising tools (nanoparticles, PGPRs) for a more efficient use of this hydroponic-based cultivation approach. Nonetheless, a better knowledge concerning the processes underpinning the acquisition of nutrients and their allocation in the different tissues, also in the presence of these promising tools, is of fundamental importance. Moreover, in a context of smart agriculture strategies application, a consistent and pertinent design of the features of the Information System with the related hardware (sensors) and software (algorithms) components is crucial. Perhaps, borrowing these tools from the industrial environment, where the new paradigm of Industry 4.0 is already applied, could be strategic. Moreover, from the practical point of view, the application of this smart approach in the hydroponic production system will unavoidably require a decoupling of the hardware component management (sensors and data loggers, connections, actuators – in charge to the farmer) from that of all the software components (maintenance of database structures/persistency, interpretation algorithms, controlling data consistency, reporting updates). In fact, the complexity of this latter task requires – at least until when a massive employment of native digitals in the agriculture context is achieved – the presence of a service center specialized in smart agriculture.
Overall, the qualitative management of the crop through the nutrient solution is therefore a concrete strategy, already applicable and characterized by completely new perspectives that will help overcoming the current limits.
Author Contributions
All the authors have equally contributed to the preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work has been financially supported by the Free University of Bozen – Bolzano (TN2053, TN2071, and TN2081).
- Ahmed A. F., Yu H., Yang X., Jiang W. (2014). Deficit irrigation affects growth, yield, vitamin C content, and irrigation water use efficiency of hot pepper grown in soilless culture. HortScience 49, 722–728. 10.21273/HORTSCI.49.6.722 [ DOI ] [ Google Scholar ]
- Alegria Terrazas R., Giles C., Paterson E., Robertson-Albertyn S., Cesco S., Mimmo T., et al. (2016). Plant-microbiota interactions as a driver of the mineral turnover in the rhizosphere. Adv. Appl. Microbiol. 95, 1–67. 10.1016/bs.aambs.2016.03.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- Amalfitano C., Del Vacchio L., Somma S., Cuciniello A., Caruso G. (2017). Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of “Friariello” pepper grown in hydroponics. Hortic. Sci. 44, 91–98. 10.17221/172/2015-HORTSCI [ DOI ] [ Google Scholar ]
- Amooaghaie R. (2011). Fungal disinfection by nanofiltration in tomato soilless culture. World Acad. Sci. Eng. Technol. 5, 454–456. 10.5281/zenodo.1078324 [ DOI ] [ Google Scholar ]
- Asaduzzaman M., Asao T. (2012). Autotoxicity in beans and their allelochemicals. Sci. Hortic. 134, 26–31. 10.1016/j.scienta.2011.11.035 [ DOI ] [ Google Scholar ]
- Asaduzzaman M., Kobayashi Y., Isogami K., Tokura M., Tokumasa K., Asao T. (2012). Growth and yield recovery in strawberry plants under autotoxicity through electrodegradation. Eur. J. Hortic. Sci. 77, 58–67. ISSN: 1611-4426. [ Google Scholar ]
- Asher C. J., Edwards D. G. (1983). “Modern solution culture techniques” in Inorganic plant nutrition. eds. Pirson A., Zimmermann M. H. (Berlin, Heidelberg: Springer; ), 94–119. [ Google Scholar ]
- Astolfi S., Ortolani M. R., Catarcione G., Paolacci A. R., Cesco S., Pinton R., et al. (2014). Cadmium exposure affects iron acquisition in barley ( Hordeum vulgare ) seedlings. Physiol. Plant. 152, 646–659. 10.1111/ppl.12207, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Astolfi S., Zuchi S., Neumann G., Cesco S., di Toppi L. S., Pinton R. (2012). Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation. J. Exp. Bot. 63, 1241–1250. 10.1093/jxb/err344, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Ávila F. W., Yang Y., Faquin V., Ramos S. J., Guilherme L. R. G., Thannhauser T. W., et al. (2014). Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem. 165, 578–586. 10.1016/j.foodchem.2014.05.134 [ DOI ] [ PubMed ] [ Google Scholar ]
- Bailey B. J., Haggett B. G. D., Hunter A., Albery W. J., Svanberg L. R. (1988). Monitoring nutrient film solutions using ion-selective electrodes. J. Agric. Eng. Res. 40, 129–142. 10.1016/0021-8634(88)90110-2 [ DOI ] [ Google Scholar ]
- Bamsey M., Graham T., Thompson C., Berinstain A., Scott A., Dixon M. (2012). Ion-specific nutrient management in closed systems: the necessity for ion-selective sensors in terrestrial and space-based agriculture and water management systems. Sensors 12, 13349–13392. 10.3390/s121013349, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barbosa G. L., Almeida Gadelha F. D., Kublik N., Proctor A., Reichelm L., Weissinger E., et al. (2015). Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 12, 6879–6891. 10.3390/ijerph120606879, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bar-Yosef B. (2008). “Fertigation management and crops response to solution recycling in semi-closed greenhouses” in Soilless culture: Theory and practice. eds. Raviv M., Lieth H. J. (Amsterdam, The Netherlands: Elsevier; ), 341–424. [ Google Scholar ]
- Baset Mia M. A., Shamsuddin Z. H., Wahab Z., Marziah M. (2010). Effect of plant growth promoting rhizobacterial (PGPR) inoculation on growth and nitrogen incorporation of tissue-cultured Musa plantlets under nitrogen-free hydroponics condition. Aust. J. Crop. Sci. 4, 85–90. Available at: http://www.cropj.com/mia_4_2_2010_85_90.pdf [ Google Scholar ]
- Bashan Y. (1990). Short exposure to Azospirillum brasilense Cd inoculation enhanced proton efflux of intact wheat roots. Can. J. Microbiol. 36, 419–425. 10.1139/m90-073 [ DOI ] [ Google Scholar ]
- Bashan Y., Levanony H., Mitiku G. (1989). Changes in proton efflux of intact wheat roots induced by Azospirillum brasilense Cd. Can. J. Microbiol. 35, 691–697. 10.1139/m89-113 [ DOI ] [ Google Scholar ]
- Benke K., Tomkins B. (2017). Future food-production systems: vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 13, 13–26. 10.1080/15487733.2017.1394054 [ DOI ] [ Google Scholar ]
- Bertrand H., Plassard C., Pinochet X., Touraine B., Normand P., Cleyet-Marel J. C. (2000). Stimulation of the ionic transport system in Brassica napus by a plant growth-promoting rhizobacterium ( Achromobacter sp.). Can. J. Microbiol. 46, 229–236. 10.1139/w99-137, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Bloom A. J., Jackson L. E., Smart D. R. (1993). Root growth as a function of ammonium and nitrate in the root zone. Plant Cell Environ. 16, 199–206. 10.1111/j.1365-3040.1993.tb00861.x [ DOI ] [ Google Scholar ]
- Bradley P., Marulanda C. (2001). Simplified hydroponics to reduce global hunger. Acta Hortic. 554, 289–295. Available at: https://www.scopus.com/inward/record.uri?eid=2-s2.0-60849112730&partnerID=40&md5=acf7509279425410e4536d6658aab38b [ Google Scholar ]
- Burman U., Saini M., Kumar P. (2013). Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 95, 605–612. 10.1080/02772248.2013.803796 [ DOI ] [ Google Scholar ]
- Bybordi A., Tabatabaei S. J., Ahmadov A. (2012). Influence of salinity and ammonium:nitrate ratio on growth, photosynthesis, fatty acid and the activity of antioxidative enzymes in canola. J. Plant Nutr. 35, 2089–2106. 10.1080/01904167.2012.723772 [ DOI ] [ Google Scholar ]
- Calcante A., Mazzetto F. (2014). Design, development and evaluation of a wireless system for the automatic identification of implements. Comput. Electron. Agric. 101, 118–127. 10.1016/j.compag.2013.12.010 [ DOI ] [ Google Scholar ]
- Campos E. V. R., de Oliveira J. L., Fraceto L. F. (2014). Applications of controlled release systems for fungicides, herbicides, acaricides, nutrients, and plant growth hormones: a review. Adv. Sci. Eng. Med. 6, 373–387. 10.1166/asem.2014.1538 [ DOI ] [ Google Scholar ]
- Canellas L., Balmori D., Médici L., Aguiar N., Campostrini E., Rosa R. C., et al. (2013). A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize ( Zea mays L.). Plant Soil 366, 119–132. 10.1007/s11104-012-1382-5 [ DOI ] [ Google Scholar ]
- Canellas L. P., Olivares F. L., Okorokova-fac A. L. (2002). Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H + -ATPase activity in maize roots. Plant Physiol. 130, 1951–1957. 10.1104/pp.007088, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carmassi G., Incrocci L., Maggini R., Malorgio F., Tognoni F., Pardossi A. (2005). Modeling salinity build-up in recirculating nutrient solution culture. J. Plant Nutr. 28, 431–445. 10.1081/PLN-200049163 [ DOI ] [ Google Scholar ]
- Cesco S., Römheld V., Varanini Z., Pinton R. (2000). Solubilization of iron by water-extractable humic substances. J. Plant Nutr. Soil Sci. 163, 285–290. [ DOI ] [ Google Scholar ]
- Charumathi S., Kaviya R. M., Kumariyarasi J., Manisha R., Dhivya P. (2017). Optimization and control of hydroponics agriculture using IOT. Asian J. Appl. Sci. Technol. 1, 96–98. [ Google Scholar ]
- Chekli L., Kim J. E., El Saliby I., Kim Y., Phuntsho S., Li S., et al. (2017). Fertilizer drawn forward osmosis process for sustainable water reuse to grow hydroponic lettuce using commercial nutrient solution. Sep. Purif. Technol. 181, 18–28. 10.1016/j.seppur.2017.03.008 [ DOI ] [ Google Scholar ]
- Chen J. (2007). Rapid urbanization in China: a real challenge to soil protection and food security. Catena 69, 1–15. 10.1016/j.catena.2006.04.019 [ DOI ] [ Google Scholar ]
- Chen F., Wei D., Tang Y. (2011). Virtual ion selective electrode for online measurement of nutrient solution components. IEEE Sensors J. 11, 462–468. 10.1109/JSEN.2010.2060479 [ DOI ] [ Google Scholar ]
- Cho W. J., Kim H.-J., Jung D. H., Kang C. I., Choi G.-L., Son J.-E. (2017). An embedded system for automated hydroponic nutrient solution management. Trans. ASABE 60, 1083–1096. 10.13031/trans.12163 [ DOI ] [ Google Scholar ]
- Colombo C., Palumbo G., He J.-Z., Pinton R., Cesco S. (2013). Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J. Soils Sediments 14, 538–548. 10.1007/s11368-013-0814-z [ DOI ] [ Google Scholar ]
- Connolly E. L., Campbell N. H., Grotz N., Prichard C. L., Guerinot M. L. (2003). Overexpression of the FRO2 Ferric Chelate Reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 133, 1102–1110. 10.1104/pp.103.025122, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- D’Imperio M., Renna M., Cardinali A., Buttaro D., Santamaria P., Serio F. (2016a). Silicon biofortification of leafy vegetables and its bioaccessibility in the edible parts. J. Sci. Food Agric. 96, 751–756. 10.1002/jsfa.7142 [ DOI ] [ PubMed ] [ Google Scholar ]
- D’Imperio M., Renna M., Cardinali A., Buttaro D., Serio F., Santamaria P. (2016b). Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 213, 149–156. 10.1016/j.foodchem.2016.06.071 [ DOI ] [ PubMed ] [ Google Scholar ]
- da Silva Cuba Carvalho R., Bastos R. G., Souza C. F. (2018). Influence of the use of wastewater on nutrient absorption and production of lettuce grown in a hydroponic system. Agric. Water Manag. 203, 311–321. 10.1016/j.agwat.2018.03.028 [ DOI ] [ Google Scholar ]
- De Rijck G., Schrevens E. (1998a). Elemental bioavailability in nutrient solutions in relation to complexation reactions. J. Plant Nutr. 21, 849–859. 10.1080/01904169809365448 [ DOI ] [ Google Scholar ]
- De Rijck G., Schrevens E. (1998b). Elemental bioavailability in nutrient solutions in relation to precipitation reactions. J. Plant Nutr. 21, 2103–2113. 10.1080/01904169809365547 [ DOI ] [ Google Scholar ]
- de Santiago A., García-López A. M., Quintero J. M., Avilés M., Delgado A. (2013). Effect of Trichoderma asperellum strain T34 and glucose addition on iron nutrition in cucumber grown on calcareous soils. Soil Biol. Biochem. 57, 598–605. 10.1016/j.soilbio.2012.06.020 [ DOI ] [ Google Scholar ]
- De Silva P. C. P., De Silva P. C. A. (2016). “Ipanera: an industry 4.0 based architecture for distributed soil-less food production systems” in 2016 Manufacturing & Industrial Engineering Symposium (MIES), (Colombo, Sri Lanka: IEEE: ), 1–5. [ Google Scholar ]
- Dinesh K., Gobinath M., Subathra M. (2018). “A survey on intelligent internet of things-technology and its application” in International Conference on Inventive Research in Computing Applications (ICIRCA), (Coimbatore, India: ), 81–84. [ Google Scholar ]
- Dukes M. D., Zotarelli L., Liu G. D., Simonne E. H. (2012). Principles and practices of irrigation management for vegetables.
- Ehret D. L., Alsanius B., Wohanka W., Menzies J. G., Utkhede R. (2001). Disinfestation of recirculating nutrient solutions in greenhouse horticulture. Agronomie 21, 323–339. 10.1051/agro:2001127 [ DOI ] [ Google Scholar ]
- El Kassis E., Cathala N., Rouached H., Fourcroy P., Berthomieu P., Terry N., et al. (2007). Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 143, 1231–1241. 10.1104/pp.106.091462 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Elmer W. H., White J. C. (2016). The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano 3, 1072–1079. 10.1039/C6EN00146G [ DOI ] [ Google Scholar ]
- El-Sayed S. F., Hassan H. A., Mahmoud S. O. (2015). Effect of some soilless culture techniques on sweet pepper growth, production, leaves chemical contents and water consumption under greenhouse conditions. Middle East J. Agric. Res., 682–691. ISSN: 2077-4605. [ Google Scholar ]
- Errebhi M., Wilcox G. E. (1990). Plant species response to ammonium-nitrate concentration ratios. J. Plant Nutr. 13, 1017–1029. 10.1080/01904169009364132 [ DOI ] [ Google Scholar ]
- Fageria N. K. (2010). The use of nutrients in crop plants. Broken Sound Parakway NW, Suite, Boca Raton: CRC Press. [ Google Scholar ]
- Fallovo C., Rouphael Y., Rea E., Battistelli A., Colla G. (2009). Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 89, 1682–1689. 10.1002/jsfa.3641 [ DOI ] [ Google Scholar ]
- Fazlil Ilahi W. F., Ahmad D., Husain M. C. (2017). Effects of root zone cooling on butterhead lettuce grown in tropical conditions in a coir-perlite mixture. Hortic. Environ. Biotechnol. 58, 1–4. 10.1007/s13580-017-0123-3 [ DOI ] [ Google Scholar ]
- Ferrarese M., Sourestani M., Quattrini E., Schiavi M., Ferrante A. (2012). Biofortification of spinach plants applying selenium in the nutrient solution of floating system. Veg. Crop. Res. Bull. 76, 127–136. 10.2478/v10032-012-0009-y [ DOI ] [ Google Scholar ]
- Forde B. G., Clarkson D. T. (1999). Nitrate and ammonium nutrition of plants: physiological and molecular perspectives. Adv. Bot. Res. 30, 1–90. 10.1016/S0065-2296(08)60226-8 [ DOI ] [ Google Scholar ]
- García J. A. L., Probanza A., Ramos B., Palomino M. R., Gutiérrez Mañero F. J. (2004). Effect of inoculation of Bacillus licheniformis on tomato and pepper. Agronomie 24, 169–176. 10.1051/agro:2004020 [ DOI ] [ Google Scholar ]
- Gardea-Torresdey J. L., Rico C. M., White J. C. (2014). Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ. Sci. Technol. 48, 2526–2540. 10.1021/es4050665, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Garibaldi A., Gilardi G., Cogliati E. E., Gullino M. L. (2012). Silicon and increased electrical conductivity reduce downy mildew of soilless grown lettuce. Eur. J. Plant Pathol. 132, 123–132. 10.1007/s10658-011-9855-6 [ DOI ] [ Google Scholar ]
- Gierth M., Mäser P. (2007). Potassium transporters in plants – involvement in K + acquisition, redistribution and homeostasis. FEBS Letters 581, 2348–2356. 10.1016/j.febslet.2007.03.035, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Giro A., Ferrante A. (2017). Postharvest physiology of Corchorus olitorius baby leaf growing with different nutrient solutions. J. Hortic. Sci. Biotechnol. 93, 1–9. 10.1080/14620316.2017.1382313 [ DOI ] [ Google Scholar ]
- Giuffrida F., Cassaniti C., Malvuccio A., Leonardi C. (2017). Effects of salt stress imposed during two growth phases on cauliflower production and quality. J. Sci. Food Agric. 97, 1552–1560. 10.1002/jsfa.7900 [ DOI ] [ PubMed ] [ Google Scholar ]
- Giuffrida F., Graziani G., Fogliano V., Scuderi D., Romano D., Leonardi C. (2014). Effects of nutrient and NaCl salinity on growth, yield, quality and composition of pepper grown in soilless closed system. J. Plant Nutr. 37, 1455–1474. 10.1080/01904167.2014.881874 [ DOI ] [ Google Scholar ]
- Glick B. R. (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012, 1–15. 10.6064/2012/963401, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gonzalo M. J., Lucena J. J., Hernández-Apaolaza L. (2013). Effect of silicon addition on soybean ( Glycine max ) and cucumber ( Cucumis sativus ) plants grown under iron deficiency. Plant Physiol. Biochem. 70, 455–461. 10.1016/j.plaphy.2013.06.007, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Gottardi S., Iacuzzo F., Tomasi N., Cortella G., Manzocco L., Pinton R., et al. (2012). Beneficial effects of silicon on hydroponically grown corn salad ( Valerianella locusta (L.) Laterr) plants. Plant Physiol. Biochem. 56, 14–23. 10.1016/j.plaphy.2012.04.002, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Grattan S., Grieve C. (1998). Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 78, 127–157. 10.1016/S0304-4238(98)00192-7 [ DOI ] [ Google Scholar ]
- Gruda N. (2009). Do soilless culture systems have an influence on product quality of vegetables? J. Appl. Bot. Food Qual. 82, 141–147. [ Google Scholar ]
- Gül A., Kidoglu F., Tüzel Y. (2008). Effects of nutrition and Bacillus amyloliquefaciens on tomato ( Solanum lycopersicum L.) growing in perlite. Span. J. Agric. Res. 6, 422–429. 10.5424/sjar/2008063-335 [ DOI ] [ Google Scholar ]
- Gupta M., Qiu X., Wang L., Xie W., Zhang C., Xiong L., et al. (2008). KT/HAK/KUP potassium transporters gene family and their whole-life cycle expression profile in rice ( Oryza sativa ). Mol. Gen. Genomics. 280, 437–452. 10.1007/s00438-008-0377-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- Gustafsson J. P. (2013). Visual MINTEQ ver. 3.1.
- Gutiérrez M., Alegret S., Cáceres R., Casadesús J., Marfà O., del Valle M. (2007). Application of a potentiometric electronic tongue to fertigation strategy in greenhouse cultivation. Comput. Electron. Agric. 57, 12–22. 10.1016/j.compag.2007.01.012 [ DOI ] [ Google Scholar ]
- Gutiérrez M., Alegret S., Cáceres R., Casadesús J., Marfà O., del Valle M. (2008). Nutrient solution monitoring in greenhouse cultivation employing a potentiometric electronic tongue. J. Agric. Food Chem. 56, 1810–1817. 10.1021/jf073438s, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Haghighi M., Abolghasemi R., Teixeira da Silva J. A. (2014). Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci. Hortic. 178, 231–240. 10.1016/J.SCIENTA.2014.09.006 [ DOI ] [ Google Scholar ]
- Haghighi M., Pessarakli M. (2013). Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes ( Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 161, 111–117. 10.1016/j.scienta.2013.06.034 [ DOI ] [ Google Scholar ]
- Hamdy A., Ahmed F. T., Choukr-Allah R. (2002). Muskmelon production in soilless culture under saline irrigation practices and soil conditioner application. Acta Hortic., 321–330. 10.17660/actahortic.2002.573.37 [ DOI ] [ Google Scholar ]
- Haynes R. J., Goh K. M. (1978). Ammonium and nitrate nutrition of plants. Biol. Rev. 53, 465–510. 10.1111/j.1469-185X.1978.tb00862.x [ DOI ] [ Google Scholar ]
- He X. L., Fan S. K., Zhu J., Guan M. Y., Liu X. X., Zhang Y. S., et al. (2017). Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil 416, 453–462. 10.1007/s11104-017-3232-y [ DOI ] [ Google Scholar ]
- Hibar K., Daami-Remadi M., Hamada W., El-Mahjoub M. (2006). Bio-fungicides as an alternative for tomato Fusarium crown and root rot control. Tunis. J. Plant Prot. 1, 19–29. [ Google Scholar ]
- Hickman G. (2016). International greenhouse vegetable production – Statistics. Mariposa, CA, USA: Cuesta Roble Greenhouse Vegetable Consulting. [ Google Scholar ]
- Hopper J. L., Parker D. R. (1999). Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 210, 199–207. 10.1023/A:1004639906245 [ DOI ] [ Google Scholar ]
- Hosseinzadeh S., Liu Z., De Graeve J., Kheet M. B., Libbrecht W., De Clercq J., et al. (2019). Recirculating water treatment in closed hydroponic systems: assessment of granular activated carbon and soft templated mesoporous carbon for adsorptive removal of root exudates. Environ. Processes 6, 1–23. 10.1007/s40710-019-00347-0 [ DOI ] [ Google Scholar ]
- Hosseinzadeh S., Verheust Y., Bonarrigo G., Van Hulle S. (2017). Closed hydroponic systems: operational parameters, root exudates occurrence and related water treatment. Rev. Environ. Sci. Biotechnol. 16, 59–79. 10.1007/s11157-016-9418-6 [ DOI ] [ Google Scholar ]
- Islam M. Z., Mele M. A., Baek J. P., Kang H. (2018). Iron, iodine and selenium effects on quality, shelf life and microbial activity of cherry tomatoes. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 46, 388–392. 10.1186/s41182-018-0085-x, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jeyasubramanian K., Gopalakrishnan Thoppey U. U., Hikku G. S., Selvakumar N., Subramania A., Krishnamoorthy K. (2016). Enhancement in growth rate and productivity of spinach grown in hydroponics with iron oxide nanoparticles. RSC Adv. 6, 15451–15459. 10.1039/C5RA23425E [ DOI ] [ Google Scholar ]
- Jones J. B., Zitter T. A., Momol T. M., Miller S. A. (1991). Compendium of tomato diseases and pests. The American Phytopathological Society. [ Google Scholar ]
- Jung D. H., Kim H.-J., Choi G. L., Ahn T.-I., Son J.-E., Sudduth K. A. (2015). Automated lettuce nutrient solution management using an array of ion-selective electrodes. Trans. ASABE 58, 1309–1319. 10.13031/trans.58.11228 [ DOI ] [ Google Scholar ]
- Kagermann H., Wahlster W., Helbig J. (2013). Umsetzungsempfehlungen für das Zukunftsprojekt Industrie 4.0. Abschlussbericht des Arbeitskreises Industrie 4.0. Frankfurt: Promotorengruppe Kommunikation der Forschungsunion Wirtschaft – Wissenschaft, acatech. [ Google Scholar ]
- Kalteh M., Alipour Z. T., Ashraf S., Marashi Aliabadi M., Falah Nosratabadi A. (2018). Effect of silica nanoparticles on basil ( Ocimum basilicum ) under salinity stress. J. Chem. Health Risks 4, 49–55. 10.22034/JCHR.2018.544075 [ DOI ] [ Google Scholar ]
- Karuppanapandian T., Wang H. W., Prabakaran N., Jeyalakshmi K., Kwon M., Manoharan K., et al. (2011). 2,4-dichlorophenoxyacetic acid-induced leaf senescence in mung bean ( Vigna radiata L. Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiol. Biochem. 49, 168–177. 10.1016/j.plaphy.2010.11.007, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Khan M. N., Mobin M., Abbas Z. K., AlMutairi K. A., Siddiqui Z. H. (2017). Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 110, 194–209. 10.1016/j.plaphy.2016.05.038, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Kim H.-J., Kim W.-K., Roh M.-Y., Kang C.-I., Park J.-M., Sudduth K. A. (2013). Automated sensing of hydroponic macronutrients using a computer-controlled system with an array of ion-selective electrodes. Comput. Electron. Agric. 93, 46–54. 10.1016/j.compag.2013.01.011 [ DOI ] [ Google Scholar ]
- Kinoshita T., Yamazaki H., Inamoto K., Yamazaki H. (2016). Analysis of yield components and dry matter production in a simplified soilless tomato culture system by using controlled-release fertilizers during summer-winter greenhouse production. Sci. Hortic. 202, 17–24. 10.1016/j.scienta.2016.02.019 [ DOI ] [ Google Scholar ]
- Kitazawa H., Asao T., Ban T., Pramanik M. H. R., Hosoki T. (2005). Autotoxicity of root exudates from strawberry in hydroponic culture. J. Hortic. Sci. Biotechnol. 80, 677–680. 10.1080/14620316.2005.11511997 [ DOI ] [ Google Scholar ]
- Korshunova Y., Eide D., Gregg Clark W., Lou Guerinot M., Pakrasi H. (1999). The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 40, 37–44. 10.1023/A:1026438615520, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Kraemer S. M., Crowley D. E., Kretzschmar R. (2006). Geochemical aspects of phytosiderophore-promoted iron acquisition by plants. Adv. Agron. 91, 1–46. 10.1016/S0065-2113(06)91001-3 [ DOI ] [ Google Scholar ]
- Kronzucker H. J., Glass A. D. M., Siddiqi M. Y. (1999). Inhibition of nitrate uptake by ammonium in barley. Analysis of component fluxes. Plant Physiol. 120, 283–292. 10.1104/pp.120.1.283, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee S., Lee J. (2015). Beneficial bacteria and fungi in hydroponic systems: types and characteristics of hydroponic food production methods. Sci. Hortic. 195, 206–215. 10.1016/j.scienta.2015.09.011 [ DOI ] [ Google Scholar ]
- Lee J. Y., Rahman A., Azam H., Kim H. S., Kwon M. J. (2017). Characterizing nutrient uptake kinetics for efficient crop production during Solanum lycopersicum var. cerasiforme Alef. growth in a closed indoor hydroponic system. PLoS One 12:e0177041. 10.1371/journal.pone.0177041, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee Y. D., Takakura T. (1995). Root cooling for spinach in deep hydroponic culture under high air temperature conditions. Acta Hortic. 399, 121–126. 10.17660/ActaHortic.1995.399.12 [ DOI ] [ Google Scholar ]
- Li R., Li D.-W., Yan A.-L., Hong C.-L., Liu H.-P., Pan L.-H., et al. (2017). The bioaccessibility of iodine in the biofortified vegetables throughout cooking and simulated digestion. J. Food Sci. Technol. 55, 366–375. 10.1007/s13197-017-2946-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Liang Y., Nikolic M., Bélanger R., Gong H., Song A. (2015). Silicon in agriculture: From theory to practice. Netherlands: Springer. [ Google Scholar ]
- Lin D., Xing B. (2007). Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 150, 243–250. 10.1016/j.envpol.2007.01.016, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Liu R., Lal R. (2015). Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 514, 131–139. 10.1016/j.scitotenv.2015.01.104 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lucena J. J. (2003). Fe chelates for remediation of Fe chlorosis in strategy I plants. J. Plant Nutr. 26, 1969–1984. 10.1081/PLN-120024257 [ DOI ] [ Google Scholar ]
- Lugtenberg B., Kamilova F. (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63, 541–556. 10.1146/annurev.micro.62.081307.162918, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Ma J. F. (2004). Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 50, 11–18. 10.1080/00380768.2004.10408447 [ DOI ] [ Google Scholar ]
- Ma J., Takahashi E. (1989). Effect of silicic acid on phosphorus uptake by rice plant. Soil Sci. Plant Nutr. 35, 227–234. 10.1080/00380768.1989.10434755 [ DOI ] [ Google Scholar ]
- Malagoli M., Schiavon M., Dall’Acqua S., Pilon-Smits E. A. H. (2015). Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 6:280. 10.3389/fpls.2015.00280, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marschner P. (2012). Marschner’s mineral nutrition of higher plants. 3rd Edn. London: Academic Press. [ Google Scholar ]
- Martínez C. E., McBride M. B. (2000). Aging of coprecipitated Cu in alumina: changes in structural location, chemical form, and solubility. Geochim. Cosmochim. Acta 64, 1729–1736. 10.1016/S0016-7037(00)00344-6 [ DOI ] [ Google Scholar ]
- Massa D., Incrocci L., Maggini R., Carmassi G., Campiotti C. A., Pardossi A. (2010). Strategies to decrease water drainage and nitrate emission from soilless cultures of greenhouse tomato. Agric. Water Manage. 97, 971–980. 10.1016/j.agwat.2010.01.029 [ DOI ] [ Google Scholar ]
- Maucieri C., Nicoletto C., Junge R., Schmautz Z., Sambo P., Borin M. (2017). Hydroponic systems and water management in aquaponics: a review. Ital. J. Agron. 11, 1–31. 10.4081/ija.2017.1012 [ DOI ] [ Google Scholar ]
- Mazzetto F., Gallo R., Importuni P., Petrera S., Sacco P. (2017). Automatic filling of field activities register, from challenge into reality. Chem. Eng. Trans. 58, 667–672. 10.3303/CET1758112 [ DOI ] [ Google Scholar ]
- McBride M. B. (1994). Environmental chemistry of soils. New York, USA: Oxford University Press. [ Google Scholar ]
- Melzer K., Bhatt V. D., Schuster T., Jaworska E., Maksymiuk K., Michalska A., et al. (2016). “Multi ion-sensor arrays: towards an electronic tongue” in 16th International Conference on Nanotechnology – IEEE NANO 2016, 475–478.
- Mengel K., Viro M., Hehl G. (1976). Effect of potassium on uptake and incorporation of ammonium-nitrogen of rice plants. Plant Soil 44, 547–558. 10.1007/BF00011374 [ DOI ] [ Google Scholar ]
- Meško M., Suklan J., Roblek V. (2017). “The impact of the Internet of Things to value added in knowledge-intensive organizations” in Knowledge management strategies and applications (InTech; ). [ Google Scholar ]
- Mimmo T., Del Buono D., Terzano R., Tomasi N., Vigani G., Crecchio C., et al. (2014). Rhizospheric organic compounds in the soil-microorganism-plant system: their role in iron availability. Eur. J. Soil Sci. 65, 629–642. 10.1111/ejss.12158 [ DOI ] [ Google Scholar ]
- Mimmo T., Tiziani R., Valentinuzzi F., Lucini L., Nicoletto C., Sambo P., et al. (2017). Selenium biofortification in Fragaria × ananassa : implications on strawberry fruits quality, content of bioactive health beneficial compounds and metabolomic profile. Front. Plant Sci. 8:1887 Available at: https://www.frontiersin.org/article/10.3389/fpls.2017.01887 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miyama Y., Sunada K., Fujiwara S., Hashimoto K. (2009). Photocatalytic treatment of waste nutrient solution from soil-less cultivation of tomatoes planted in rice hull substrate. Plant Soil 318, 275–283. 10.1007/s11104-008-9837-4 [ DOI ] [ Google Scholar ]
- Mohammadi R., Maali-Amiri R., Abbasi A. (2013). Effect of TiO 2 nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 152, 403–410. 10.1007/s12011-013-9631-x, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Mondal M. F., Asaduzzaman M., Kobayashi Y., Ban T., Asao T. (2013). Recovery from autotoxicity in strawberry by supplementation of amino acids. Sci. Hortic. 164, 137–144. 10.1016/j.scienta.2013.09.019 [ DOI ] [ Google Scholar ]
- Mondal M. F., Asaduzzaman M., Tanaka H., Asao T. (2015). Effects of amino acids on the growth and flowering of Eustoma grandiflorum under autotoxicity in closed hydroponic culture. Sci. Hortic. 192, 453–459. 10.1016/j.scienta.2015.05.024 [ DOI ] [ Google Scholar ]
- Montesano F. F., D’Imperio M., Parente A., Cardinali A., Renna M., Serio F. (2016). Green bean biofortification for Si through soilless cultivation: plant response and Si bioaccessibility in pods. 6. 10.1038/srep31662, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morimoto T., Hashimoto Y. (1996). Optimal control of plant growth in hydroponics using neural network and genetic algorithms. Acta Hortic., 433–440. 10.17660/ActaHortic.1996.406.43 [ DOI ] [ Google Scholar ]
- Müller B. (2015). ChemEQL ver. 3.2.
- Müller C., Elliott J., Chryssanthacopoulos J., Arneth A., Balkovic J., Ciais P. (2017). Global gridded crop model evaluation: benchmarking, skills, deficiencies and implications. Geosci. Model Dev. Discuss. 10, 1403–1422. 10.5194/gmd-2016-207 [ DOI ] [ Google Scholar ]
- Na L., Li Z., Xiangxiang M., Ara N., Jinghua Y., Mingfang Z. (2014). Effect of nitrate/ammonium ratios on growth, root morphology and nutrient elements uptake of watermelon ( Citrullus lanatus ) seedlings. J. Plant Nutr. 37, 1859–1872. 10.1080/01904167.2014.911321 [ DOI ] [ Google Scholar ]
- Nacry P., Bouguyon E., Gojon A. (2013). Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 270, 1–29. 10.1007/s11104-013-1645-9 [ DOI ] [ Google Scholar ]
- Nair R., Varghese S. H., Nair B. G., Maekawa T., Yoshida Y., Kumar D. S. (2010). Nanoparticulate material delivery to plants. Plant Sci. 179, 154–163. 10.1016/J.PLANTSCI.2010.04.012 [ DOI ] [ Google Scholar ]
- Nancy D., Arulselvi P. I. (2014). Effect of Selenium fortification on biochemical activities of tomato ( Solanum lycopersicum ) plants. Indo Am. J. Pharm. Res. 4, 3997–4005. Available at: www.iajpr.comwww.iajpr.com (Accessed April 10, 2019). [ Google Scholar ]
- National Research Council (1997). Precision agriculture in the 21st century: Geospatial and information technologies in crop management. Washington, D.C. National Academies Press. [ Google Scholar ]
- Neocleous D., Ntatsi G., Savvas D. (2017). Physiological, nutritional and growth responses of melon ( Cucumis melo L.) to a gradual salinity built-up in recirculating nutrient solution. J. Plant Nutr. 40, 2168–2180. 10.1080/01904167.2017.1346673 [ DOI ] [ Google Scholar ]
- Neumann G. (2018). Knowledge management 4.0 – implications of the fourth industrial revolution on knowledge management in supply chains. Theory Appl. Knowl. Econ. 452. [ Google Scholar ]
- Nikolic M., Cesco S., Römheld V., Varanini Z., Pinton R. (2007). Short-term interactions between nitrate and iron nutrition in cucumber. Funct. Plant Biol. 34, 402–408. 10.1071/FP07022 [ DOI ] [ PubMed ] [ Google Scholar ]
- Packter A. (1974). The precipitation of calcium sulphate dihydrate from aqueous solution: induction periods, crystal numbers and final size. J. Cryst. Growth 21, 191–194. 10.1016/0022-0248(74)90004-9 [ DOI ] [ Google Scholar ]
- Pardossi A., Falossi F., Malorgio F., Incrocci L., Bellocchi G. (2005). Empirical models of macronutrient uptake in melon plants grown in recirculating nutrient solution culture. J. Plant Nutr. 27, 1261–1280. 10.1081/PLN-120038547 [ DOI ] [ Google Scholar ]
- Parida A. K., Das A. B. (2005). Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Saf. 60, 324–349. 10.1016/j.ecoenv.2004.06.010, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Park S., Elless M. P., Park J., Jenkins A., Lim W., Chambers E., IV, et al. (2009). Sensory analysis of calcium-biofortified lettuce. Plant Biotechnol. J. 7, 106–117. 10.1111/j.1467-7652.2008.00379.x, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Pavlovic J., Samardzic J., Maksimovi V., Timotijevic G., Stevic N., Laursen K. H., et al. (2013). Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol 198, 1096–1107. 10.1111/nph.12213 [ DOI ] [ PubMed ] [ Google Scholar ]
- Phuntsho S., Shon H. K., Hong S., Lee S., Vigneswaran S., Kandasamy J. (2012). Fertiliser drawn forward osmosis desalination: the concept, performance and limitations for fertigation. Rev. Environ. Sci. Biotechnol. 11, 147–168. 10.1007/s11157-011-9259-2 [ DOI ] [ Google Scholar ]
- Pignata G., Casale M., Nicola S. (2017). “Water and nutrient supply in horticultural crops grown in soilless culture: resource efficiency in dynamic and intensive systems” in Advances in research on fertilization management of vegetable crops eds. Tei F., Nicola S., Benincasa P. (Cham: Springer; ), 183–219. [ Google Scholar ]
- Pii Y., Aldrighetti A., Valentinuzzi F., Mimmo T., Cesco S. (2019). Azospirillum brasilense inoculation counteracts the induction of nitrate uptake in maize plants. J. Exp. Bot. 70, 1313–1324. 10.1093/jxb/ery433, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Pii Y., Graf H., Valentinuzzi F., Cesco S., Mimmo T. (2018). The effects of plant growth-promoting rhizobacteria (PGPR) on the growth and quality of strawberries. Acta Hortic. 1217, 231–238. 10.17660/ActaHortic.2018.1217.29 [ DOI ] [ Google Scholar ]
- Pii Y., Marastoni L., Springeth C., Fontanella M. C., Beone G. M., Cesco S., et al. (2016). Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ. Exp. Bot. 130, 216–225. 10.1016/j.envexpbot.2016.06.011 [ DOI ] [ Google Scholar ]
- Pii Y., Mimmo T., Tomasi N., Terzano R., Cesco S., Crecchio C. (2015). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 51, 403–415. 10.1007/s00374-015-0996-1 [ DOI ] [ Google Scholar ]
- Pinton R., Cesco S., Santi S., Agnolon F., Varanini Z. (1999). Water-extractable humic substances enhance iron deficiency responses by Fe-deficient cucumber plants. Plant and Soil; 210, 145–157. 10.1023/A:1004329513498 [ DOI ] [ Google Scholar ]
- Pinton R., Cesco S., Santi S., Varanini Z. (1997). Soil humic substances stimulate proton release by intact oat seedling roots. J. Plant Nutr. 20, 857–869. 10.1080/01904169709365301 [ DOI ] [ Google Scholar ]
- Prosser I. M., Purves J. V., Saker L. R., Clarkson D. T. (2001). Rapid disruption of nitrogen metabolism and nitrate transport in spinach plants deprived of sulphate. J. Exp. Bot. 52, 113–121. 10.1093/jxb/52.354.113 [ DOI ] [ PubMed ] [ Google Scholar ]
- Putra P. A., Yuliando H. (2015). Soilless culture system to support water use efficiency and product quality: a review. Agric. Agric. Sci. Procedia 3, 283–288. 10.1016/j.aaspro.2015.01.054 [ DOI ] [ Google Scholar ]
- Qados A. M. S. A. (2015). Mechanism of nanosilicon-mediated alleviation of salinity stress in faba bean ( Vicia faba L.) plants. Am. J. Exp. Agric. 7, 78–95. Available at: https://www.cabdirect.org/cabdirect/abstract/20153160126 (Accessed May 15, 2019). [ Google Scholar ]
- Qados A., Moftah A. (2014). Influence of silicon and nano-silicon on germination, growth and yield of faba bean ( Vicia faba L.) under salt stress conditions. Am. J. Exp. Agric. 5, 509–524. 10.9734/ajea/2015/14109 [ DOI ] [ Google Scholar ]
- Ray P. P. (2017). Internet of things for smart agriculture: technologies, practices and future direction. J. Ambient Intell. Smart Environ. 9, 395–420. 10.3233/AIS-170440 [ DOI ] [ Google Scholar ]
- Rius-Ruiz F. X., Andrade F. J., Riu J., Rius F. X. (2014). Computer-operated analytical platform for the determination of nutrients in hydroponic systems. Food Chem. 147, 92–97. 10.1016/j.foodchem.2013.09.114, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Rodriguez-Ortega W. M., Martinez V., Rivero R. M., Camara-Zapata J. M., Mestre T., Garcia-Sanchez F. (2017). Use of a smart irrigation system to study the effects of irrigation management on the agronomic and physiological responses of tomato plants grown under different temperatures regimes. Agric. Water Manag. 183, 158–168. 10.1016/j.agwat.2016.07.014 [ DOI ] [ Google Scholar ]
- Roosta H. R., Safarizadeh M., Hamidpour M. (2017). Effect of humic acid contained nano-fertile fertilizer spray on concentration of some nutrient elements in two lettuce cultivars in hydroponic system. J. Sci. Technol. Greenhouse Cult. 7. 10.18869/acadpub.ejgcst.7.4.51 [ DOI ] [ Google Scholar ]
- Rouphael Y., Colla G., Cardarelli M., Fanasca S., Salerno A., Rivera C. M., et al. (2005). Water use efficiency of greenhouse summer squash in relation to the method of culture: soil vs. soilless. Acta Hortic. 81–86. 10.17660/ActaHortic.2005.697.8 [ DOI ] [ Google Scholar ]
- Rufty T. W., Jackson W. A., Raper C. D. (1982). Inhibition of nitrate assimilation in roots in the presence of ammonium: the moderating influence of potassium. J. Exp. Bot. 33, 1122–1137. 10.1093/jxb/33.6.1122 [ DOI ] [ Google Scholar ]
- Santamaria P. (2006). Nitrate in vegetables: toxicity, content, intake and EC regulation. J. Sci. Food Agric. 86, 10–17. 10.1002/jsfa.2351 [ DOI ] [ Google Scholar ]
- Savvas D. (2003). Hydroponics: A modern technology supporting the application of integrated crop management in greenhouse. Available at: www.world-food.net (Accessed April 9, 2019).
- Schiavon M., Dall’acqua S., Mietto A., Pilon-Smits E. A. H., Sambo P., Masi A., et al. (2013). Selenium fertilization alters the chemical composition and antioxidant constituents of tomato ( Solanum lycopersicon L.). J. Agric. Food Chem. 61, 10542–10554. 10.1021/jf4031822 [ DOI ] [ PubMed ] [ Google Scholar ]
- Schiavon M., Pilon-Smits E. A. H. (2017). The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology. New Phytol. 213, 1582–1596. 10.1111/nph.14378 [ DOI ] [ PubMed ] [ Google Scholar ]
- Schimansky C. (1981). Der Einfluß einiger Versuchsparameter auf das Fluxverhalten von 28 Mg bei Gerstenkeimpflanzen in Hydrokulturversuchen. Landwirtsch. Forsch. 34, 154–165. [ Google Scholar ]
- Schimel J. P., Chapin F. S. (1996). Tundra plant uptake of amino acid and NH 4 + nitrogen in situ: plants complete well for amino acid N. Ecology 77, 2142–2147. 10.2307/2265708 [ DOI ] [ Google Scholar ]
- Shafique H. A., Sultana V., Ehteshamul-Haque S., Athar M. (2016). Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 56, 221–230. 10.1515/jppr-2016-0043 [ DOI ] [ Google Scholar ]
- Shaviv A., Hagin J., Neumann P. M. (1987). Effects of a nitrification inhibitor on efficiency of nitrogen utilization by wheat and millet. Commun. Soil Sci. Plant Anal. 18, 815–833. 10.1080/00103628709367865 [ DOI ] [ Google Scholar ]
- Shibagaki N., Rose A., McDermott J. P., Fujiwara T., Hayashi H., Yoneyama T., et al. (2002). Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2 , a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 29, 475–486. 10.1046/j.0960-7412.2001.01232.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Shin R. (2014). Strategies for improving potassium use efficiency in plants. Mol. Cells 37, 575. 10.14348/molcells.2014.0141 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Siddiqui M. H., Al-Whaibi M. H., Faisal M., Al Sahli A. A. (2014). Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 33, 2429–2437. 10.1002/etc.2697 [ DOI ] [ PubMed ] [ Google Scholar ]
- Silberbush M., Ben-Asher J. (1989). The effect of NaCl concentration on NO 3 − , K + and orthophosphate-P influx to peanut roots. Sci. Hortic. 39, 279–287. 10.1016/0304-4238(89)90121-0 [ DOI ] [ Google Scholar ]
- Singh H., Batish D. R., Kohli R. (1999). Autotoxicity: concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 18, 757–772. 10.1080/07352689991309478 [ DOI ] [ Google Scholar ]
- Smoleń S., Kowalska I., Czernicka M., Halka M., Kęska K., Sady W. (2016). Iodine and Selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants ( Lactuca sativa L. var. capitata). Front. Plant Sci. 7, 1–16. 10.3389/fpls.2016.01553 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smoleń S., Kowalska I., Sady W. (2014). Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 166, 9–16. 10.1016/j.scienta.2013.11.011 [ DOI ] [ Google Scholar ]
- Stamatakis A., Papadantonakis N., Lydakis-Simantiris N., Kefalas P., Savvas D. (2003). Effects of silicon and salinity on fruit yield and quality of tomato grown hydroponically. Acta Hortic. 609, 141–147. 10.17660/ActaHortic.2003.609.18 [ DOI ] [ Google Scholar ]
- Suhardiyanto H., Arif C., Setiawan I. B. (2009). Optimization of EC values of nutrient solution for tomato fruits quality in hydroponics system using artificial neural network and genetic algorithms. ITB J. Sci. 41, 38–49. 10.5614/itbj.sci.2009.41.1.3 [ DOI ] [ Google Scholar ]
- Tajudeen A. L., Taiwo O. S. (2018). Soilless farming – a key player in the realisation of “zero hunger” of the sustainable development goals in Nigeria. Int. J. Ecol. Sci. Environ. Eng. 5, 1–7. ISSN: 2375-3854. [ Google Scholar ]
- Takeno N. (2005). Atlas of Eh-pH diagrams. Intercomparison of thermodynamic databases: National Institute of Advanced Industrial Science and Technology, 285. [ Google Scholar ]
- Talukder M. R., Asaduzzaman M., Tanaka H., Asao T. (2018). Light-emitting diodes and exogenous amino acids application improve growth and yield of strawberry plants cultivated in recycled hydroponics. Sci. Hortic. 239, 93–103. 10.1016/j.scienta.2018.05.033 [ DOI ] [ Google Scholar ]
- Talukder M. R., Asaduzzaman M., Tanaka H., Asao T. (2019). Electro-degradation of culture solution improves growth, yield and quality of strawberry plants grown in closed hydroponics. Sci. Hortic. 243, 243–251. 10.1016/j.scienta.2018.08.024 [ DOI ] [ Google Scholar ]
- Tamai K., Ma J. F. (2008). Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 307, 21–27. 10.1007/s11104-008-9571-y [ DOI ] [ Google Scholar ]
- ten Hoopen F., Cuin T. A., Pedas P., Hegelund J. N., Shabala S., Schjoerring J. K., et al. (2010). Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J. Exp. Bot. 61, 2303–2315. 10.1093/jxb/erq057, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Terzano R., Cesco S., Mimmo T. (2015). Dynamics, thermodynamics and kinetics of exudates: crucial issues in understanding rhizosphere processes. Plant Soil 386, 399–406. 10.1007/s11104-014-2308-1 [ DOI ] [ Google Scholar ]
- Tilman D., Cassman K. G., Matson P. A., Naylor R., Polasky S. (2002). Agriculture sustainability and intensive production practices. Nature 418, 671–677. 10.1038/nature01014, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Tittarelli F., Båth B., Ceglie F. G., Garcia C., Möller K., Reents H. J., et al. (2016). Soil fertility management in organic greenhouses in Europe. BioGreenhouse COST Action FA 1105. 10.18174/373583 [ DOI ]
- Tomasi N., De Nobili M., Gottardi S., Zanin L., Mimmo T., Varanini Z., et al. (2013). Physiological and molecular characterization of Fe acquisition by tomato plants from natural Fe complexes. Biol. Fertil. Soils 49, 187–200. 10.1007/s00374-012-0706-1 [ DOI ] [ Google Scholar ]
- Tomasi N., Mimmo T., Terzano R., Alfeld M., Janssens K., Zanin L., et al. (2014). Nutrient accumulation in leaves of Fe-deficient cucumber plants treated with natural Fe complexes. Biol. Fertil. Soils 50, 973–982. 10.1007/s00374-014-0919-6 [ DOI ] [ Google Scholar ]
- Tomasi N., Pinton R., Dalla Costa L., Cortella G., Terzano R., Mimmo T., et al. (2015a). New “solutions” for floating cultivation system of ready-to-eat salad: a review. Trends Food Sci. Technol. 46, 267–276. 10.1016/j.tifs.2015.08.004 [ DOI ] [ Google Scholar ]
- Tomasi N., Pinton R., Gottardi S., Mimmo T., Scampicchio M., Cesco S. (2015b). Selenium fortification of hydroponically grown corn salad ( Valerianella locusta ). Crop Pasture Sci. 66, 1128–1136. 10.1071/CP14218 [ DOI ] [ Google Scholar ]
- Tomasi N., Rizzardo C., Monte R., Gottardi S., Jelali N., Terzano R., et al. (2009). Micro-analytical, physiological and molecular aspects of Fe acquisition in leaves of Fe-deficient tomato plants re-supplied with natural Fe-complexes in nutrient solution. Plant Soil 325, 25–38. 10.1007/s11104-009-0069-z [ DOI ] [ Google Scholar ]
- Tripathi D. K., Shweta, Singh S., Singh S., Pandey R., Singh V. P., et al. (2017). An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 110, 2–12. 10.1016/J.PLAPHY.2016.07.030 [ DOI ] [ PubMed ] [ Google Scholar ]
- Tripathi D. K., Singh S., Singh S., Dubey N. K., Chauhan D. K. (2016). Impact of nanoparticles on photosynthesis: challenges and opportunities. Mater. Focus 5, 405–411. 10.1166/mat.2016.1327 [ DOI ] [ Google Scholar ]
- Valentinuzzi F., Pii Y., Mimmo T., Savini G., Curzel S., Cesco S. (2018). Fertilization strategies as a tool to modify the organoleptic properties of raspberry ( Rubus idaeus L.) fruits. Sci. Hortic. 240, 205–212. 10.1016/j.scienta.2018.06.024 [ DOI ] [ Google Scholar ]
- Valentinuzzi F., Pii Y., Vigani G., Lehmann M., Cesco S., Mimmo T. (2015). Phosphorus and iron defciencies induce a metabolic reprogramming and affect the exudation traits of the woody plant Fragaria × ananassa . J. Exp. Bot. 66, 6483–6495. 10.1093/jxb/erv364 [ DOI ] [ PubMed ] [ Google Scholar ]
- Van der Bruggen B., Luis P. (2015). Forward osmosis: understanding the hype. Rev. Chem. Eng. 31, 1–12. 10.1515/revce-2014-0033 [ DOI ] [ Google Scholar ]
- Van Ginkel S. W., Igou T., Chen Y. (2017). Energy, water and nutrient impacts of California-grown vegetables compared to controlled environmental agriculture systems in Atlanta, GA. Resour. Conserv. Recycl. 122, 319–325. 10.1016/j.resconrec.2017.03.003 [ DOI ] [ Google Scholar ]
- Vardar G., Altıkatoğlu M., Ortaç D., Cemek M., Işıldak İ. (2015). Measuring calcium, potassium, and nitrate in plant nutrient solutions using ion-selective electrodes in hydroponic greenhouse of some vegetables. Biotechnol. Appl. Biochem. 62, 663–668. 10.1002/bab.1317 [ DOI ] [ PubMed ] [ Google Scholar ]
- Verones F., Pfister S., van Zelm R., Hellweg S. (2017). Biodiversity impacts from water consumption on a global scale for use in life cycle assessment. Int. J. Life Cycle Assess. 22, 1247–1256. 10.1007/s11367-016-1236-0 [ DOI ] [ Google Scholar ]
- Verweij W. (2017). CHEAQS.
- von Wirén N., Merrick M. (2004). “Regulation and function of ammonium carriers in bacteria, fungi, and plants” in Molecular mechanisms controlling transmembrane transport SE – 3 topics in current genetics. eds. Boles E., Krämer R. (Berlin/Heidelberg: Springer; ), 95–120. [ Google Scholar ]
- Weng H. X., Hong C. L., Yan A. L., Pan L. H., Qin Y. C., Bao L. T., et al. (2008). Mechanism of iodine uptake by cabbage: effects of iodine species and where it is stored. Biol. Trace Elem. Res. 125, 59–71. 10.1007/s12011-008-8155-2, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- White P. J. (2003). “Ion transport” in Encyclopaedia of applied plant sciences. eds. Thomas B., Murphy D. J., Murray B. G. (London: Academic Press; ), 625–634. [ Google Scholar ]
- Wiesner-Reinhold M., Schreiner M., Baldermann S., Schwarz D., Hanschen F. S., Kipp A. P., et al. (2017). Mechanisms of selenium enrichment and measurement in brassicaceous vegetables, and their application to human health. Front. Plant Sci. 8:1365. 10.3389/fpls.2017.01365, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wild A., Jones L. H. P., Macduff J. H. (1987). Uptake of mineral nutrients and crop growth: the use of flowing nutrient solutions. Adv. Agron. 41, 171–219. 10.1016/S0065-2113(08)60805-6 [ DOI ] [ Google Scholar ]
- Yasuor H., Tamir G., Stein A., Cohen S., Bar-Tal A., Ben-Gal A., et al. (2017). Does water salinity affect pepper plant response to nitrogen fertigation? Agric. Water Manag. 191, 57–66. 10.1016/j.agwat.2017.05.012 [ DOI ] [ Google Scholar ]
- Yolanda D., Hindersah H., Hadiatna F., Triawan M. (2016). “Implementation of real-time fuzzy logic control for NFT-based hydroponic system on Internet of Things environment” in 6th International Conference on System Engineering and Technology (ICSET). (Bandung, Indonesia: ), 153–159. [ Google Scholar ]
- Zaimenko N. V., Didyk N. P., Dzyuba O. I., Zakrasov O. V., Rositska N. V., Viter A. V. (2014). Enhancement of drought resistance in wheat and corn by nanoparticles of natural mineral analcite. Ecol. Balk. 6, 1–10. Available at: http://eb.bio.uni-plovdiv.bg (Accessed May 15, 2019). [ Google Scholar ]
- Zamboni A., Zanin L., Tomasi N., Avesani L., Pinton R., Varanini Z., et al. (2016). Early transcriptomic response to Fe supply in Fe-deficient tomato plants is strongly influenced by the nature of the chelating agent. BMC Genomics 17:35. 10.1186/s12864-015-2331-5 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zanin L., Tomasi N., Cesco S., Varanini Z., Pinton R. (2019). Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front. Plant Sci. 10:675. 10.3389/fpls.2019.00675, PMID: [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zekki H., Gauthier L., Gosselin A. (2019). Growth, productivity, and mineral composition of hydroponically cultivated greenhouse tomatoes, with or without nutrient solution recycling. J. Am. Soc. Hortic. Sci. 121, 1082–1088. 10.21273/jashs.121.6.1082 [ DOI ] [ Google Scholar ]
- Zhang F., Niu J., Zhang W., Chen X., Li C., Yuan L., et al. (2010). Potassium nutrition of crops under varied regimes of nitrogen supply. Plant Soil 335, 21–34. 10.1007/s11104-010-0323-4 [ DOI ] [ Google Scholar ]
- Zhang H., Sun Y., Xie X., Kim M.-S., Dowd S. E., Paré P. W. (2009). A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 58, 568–577. 10.1111/j.1365-313X.2009.03803.x, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhang Z., Zhang J., Chen Y., Li R., Wang H., Wei J. (2012). Genome-wide analysis and identification of HAK potassium transporter gene family in maize ( Zea mays L.). Mol. Biol. Rep. 39, 8465–8473. 10.1007/s11033-012-1700-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhao F.-J., McGrath S. P., Meharg A. A. (2010). Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 61, 535–559. 10.1146/annurev-arplant-042809-112152, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhao L., Wang F., Zhang Y., Zhang J. (2014). Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plants. J. Basic Microbiol. 54, S115–S124. 10.1002/jobm.201400148, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhu Y. G., Huang Y. Z., Hu Y., Liu Y. X. (2003). Iodine uptake by spinach ( Spinacia oleracea L.) plants grown in solution culture: effects of iodine species and solution concentrations. Environ. Int. 29, 33–37. 10.1016/S0160-4120(02)00129-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zuchi S., Cesco S., Astolfi S. (2012). High S supply improves Fe accumulation in durum wheat plants grown under Fe limitation. Environ. Exp. Bot. 77, 25–32. 10.1016/j.envexpbot.2011.11.001 [ DOI ] [ Google Scholar ]
- Zuchi S., Cesco S., Varanini Z., Pinton R., Astolfi S. (2009). Sulphur deprivation limits Fe-deficiency responses in tomato plants. Planta 230, 85–94. 10.1007/s00425-009-0919-1, PMID: [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (960.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Editorial: Nutrients Recycling in Hydroponics: Opportunities and Challenges Toward Sustainable Crop Production Under Controlled Environment Agriculture
Md asaduzzaman, toshiki asao.
- Author information
- Article notes
- Copyright and License information
Edited by: Youssef Rouphael, University of Naples Federico II, Italy
Reviewed by: Pradeep Kumar, Central Arid Zone Research Institute (ICAR), India; Nazim S. Gruda, University of Bonn, Germany
*Correspondence: Md Asaduzzaman [email protected]
This article was submitted to Crop and Product Physiology, a section of the journal Frontiers in Plant Science
Received 2021 Dec 29; Accepted 2022 Feb 2; Collection date 2022.
Keywords: nutrient recycling, plant factories, soilless culture, close-loop crop cascade, fruit qualities, wastewater reuse (WR), aquaponics
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Nutrient Recycling in Hydroponics
Hydroponics is a method of growing plants in soilless media or nutrient solution containing all essential mineral nutrients that potentially lead to yield and quality improvement (Gruda, 2009 ). In principle, nutrient solutions used in hydroponics can either be reused or discarded (Jensen, 1997 ; Nederhoff and Stanghellini, 2010 ). Nowadays, cultivation of horticultural crops including leafy and fruiting vegetables and medicinal herbs with pharmaceutical value are commercially grown in recycled (i.e., recirculating) hydroponics under controlled environments (Resh, 2012 ; Son et al., 2020 ).
In recycled hydroponics, nutrient solutions passed through the growing medium are collected into a reservoir and reused repeatedly. In this system, both water and mineral nutrients are used efficiently, therefore minimizing wastage of fertilizer and pollution of the environment. This type of hydroponic system has been widely used in controlled environment agriculture facilities including plant factories using artificial grow lights such as light-emitting diodes. Management of the hydroponic nutrient solution composition, along with the adjustment of environmental conditions may enhance the desired nutritional quality of the produce, regulate bioactive compounds, and increase antioxidants and other phytochemical content in soilless culture (Savvas et al., 2013 ; Asaduzzaman et al., 2018 ; Ciriello et al., 2021 ).
Hydroponics may however be challenged by the accumulation of root exudates that affect plant growth and reduce crop yield and quality. Lower growth and yield performance of several crops including lettuce, strawberry, several leafy vegetables, and ornamentals have been reported in recycled hydroponics (Asao et al., 2004 , 2007 ; Lee et al., 2006 ). Reduced growth and yield of crops grown in recycled hydroponics because of increased concentration of phytotoxic root exudates have been reported causing allelochemical stress in the strawberry (Kitazawa et al., 2005 ), tomato (Yu and Matsui, 1993 ), and cucumber (Yu and Matsui, 1994 ). Certain phytotoxic chemicals may have a significant impact on plant growth. Moreover, recycled nutrient solutions usually require sterilization to minimize pathogen loads. In this regard, chemicals and physical treatments such as hypochlorite, ozone, and UV-light, are being used for sterilization.
The present Research Topic collected 12 scientific contributions from the leading research groups throughout the world working on recycled hydroponics, nutrient solution management, the influence of LEDs on crop growth and physiology, salinity impact on growth and nutritional quality, wastewater based nutrient recycling, and also nutrient reclamation or re-mineralization. In addition, this Research Topic complies several aspects of controlled environment agriculture that are useful with for scientific community, and by extension workers and commercial entrepreneurs, for the understanding of nutrient recycling in hydroponics toward sustainable crop production. The original research and reviewed literature also present how the techniques of nutrient recycling, efficient use of available nutrients, quality improvement of crop produce, and nutrient recycling from renewable resources will help the development of sustainable agricultural systems.
Nutrient Solution Management in Recycled Hydroponics
Recycled hydroponics gained popularity in controlled environment agriculture leading to efficient use of costly fertilizer and a sustainable environment. Rufí-Salís et al. proposed a cascade system with a long-cycle tomato donor crop and five successive cycles of lettuce for a rooftop greenhouse. They quantified the scale between the donor and receiving crops and proposed three major ideas to optimize the nutrient flows while maintaining the yield and quality of the vegetables produced in the receiving crop. The variation of the nutrient content of the leachates produced by the donor crop was a key consideration that determines the number of plants to be planted as the receiving crop. It was found that the early stage of the donor crop could only produce 0.1 lettuces per tomato plant, with N as the limiting nutrient. On the other hand, the late stage of the donor crop was able to leach enough nutrients to feed 9 lettuces per tomato plant. However, attention must be paid to the electrical conductivity (EC) of the water flow to stay within non-harmful values. Nevertheless, the cascade system was shown to be efficient to mitigate the nutrient discharge of open systems, especially in terms of N and P to avoid eutrophication impacts in the early stage of the tomato crop. Considering the nutritional problems at the beginning of the cycle of the donor crop and the harmful salinity that can be reached at the end, future research needs to be designed to test different kinds of horticultural crops to discover possible viable combinations of donor and receiving crops.
Miller et al. reported that nutrient deficiencies in hydroponic production can also be observed due to recycling nutrient solutions. They evaluated the effects of recycling on solution EC changes, tissue nutrient concentration, canopy growth rate, plant water status, and shoot and root weight of lettuce in a greenhouse and suggest the development of optimal strategies for managing recycling nutrient solution in hydroponic production. This study indicates that continuous recycling with tap water containing moderate to high levels of alkalinity can result in an apparent increase in solution EC, nutrient deficiencies in the plants, and reduction in shoot growth, in spite of maintaining the solution EC at a target level. Results of this study also indicated that nutrient recycling significantly decreased N, P, K, and Fe and increased Na and Cu levels in the tissue, in addition to increasing solution EC between adjustments compared to the control. Through image analysis of plants reveals the negative effects of recycling on canopy area started 2 weeks after transplanting. Thus, they hypothesized that certain unwanted compounds (e.g., bicarbonates) and slowly consumed elements (e.g., Ca, Mg) were added to the recycling solution through the alkaline tap water with time.
Ahn et al. designed an EC-based nutrient recycling soilless culture system by theoretical and experimental analyses. An integrated model of solutes such as K + , Ca 2+ , and Mg 2+ and water transport in growing media, automated nutrient solution preparation, and nutrient uptake was designed. In the simulation, the intrinsic characteristics of nutrient changes among open-, semi- closed-, and closed-loop soilless cultures were compared, and stochastic simulations for nutrient control were performed in the closed-loop system. Four automated irrigation modules for comparing nutrient changes among the soilless culture systems were constructed for sweet pepper grown in the greenhouse. Theoretical and experimental analyses exhibited that nutrient variations in these culture systems can be integrated as a function of nutrient supply to the system's boundary areas. Furthermore, stochastic simulation analysis indicated that the nutrient ratio in the soilless culture system reveals the nutrient uptake parameter-based deterministic patterns. Thus, they suggested that the nutrient ratio in the closed-loop soilless culture could be controlled by the long-term feedback of this ratio.
The quality of crop produce can be improved through quantitative management of the hydroponic nutrient solution. The desired mineral content in fruits and vegetables can be either increased or decreased through their elevated or deficient concentration in the culture solution. Zhu et al. studied appropriate NH 4 + / NO 3 - ratio that triggers plant growth and nutrient uptake of flowering Chinese cabbage by optimizing the pH value of the nutrient solution. They analyzed the changes in nutrient solution composition, the content of different N forms in plant tissues and exudates, and expression of plasma membrane H + -ATPase genes under different NH 4 + / NO 3 - ratios (0/100, 10/90, 25/75, 50/50). Compared with the control, NH 4 + / NO 3 - ratios (0/100, 10/90, and 25/75) significantly reduced the NO 3 - content and increased the NH 4 + , amino acid, and soluble protein contents of flowering Chinese cabbage to varying extents. NH 4 + / NO 3 - ratio (10/90) significantly increased the N use efficiency, whereas NH 4 + / NO 3 - ratio (25/75) significantly decreased it to about 70.25% of that control. Owing to the difference in N absorption and utilization among seedlings, the pH value of the nutrient solution differed under different NH 4 + /NO 3 ratios.
Led Lighting, Nitrogen Metabolism and Environmental Controls in Recycled Hydroponics
In a plant factory with artificial lighting (PFAL), LEDs have widely been used for economic considerations and also to ensure a lower amount of heat emission inside the controlled room. The influence of LED spectrum along with the application of amino acid as nitrogen source has been studied by Talukder et al. ( 2018 ). Li et al. studied the effect of the LED spectrum on the quality and nitrogen metabolism under recycled hydroponics. They found that LED illumination spectra had a significant influence on the growth and nitrogen metabolism of lettuce. Adding green, purple, and far-red light had a negative impact on lettuce growth through decreased photosynthetic photon flux density. Purple LED supplementation was found to be conducive to vitamin-C accumulation in lettuce leaves. Adding purple light inhibited NR (nitrate reductase) and NiR (nitrite reductase) activities and caused a low nitrate, nitrite, and ammonium content while they contributed to amino acid accumulation for nitrogen assimilation. Thus, red, blue, and purple LEDs are recommended for use as supplemental lighting strategy greenhouse production.
He et al. grew purslane ( Portulaca oleracea L.) in different NaCl salinities in hydroponics under LED lightings. Greater shoot and root dry mass with higher proline and carotene concentration were observed under 100 mM NaCl than fresh water, 200 and 300 mM. However, increasing salinity levels such as 200 and 300 mM NaCl decreases the shoot and root dry mass, ascorbic acid, and total phenolic compounds under lower leaf water content and photosynthetic performance. They concluded that it is feasible to grow purslane under 100 mM NaCl to achieve higher productivity and better quality.
In controlled environment agriculture, environmental factors including VPD fluctuation greatly influence the photosynthesis and yield of a plant. Inoue et al. examined the effects of the vapor pressure deficit (VPD) fluctuation on the photosynthetic and growth parameters in lettuce. In this study, gas exchange, chlorophyll fluorescence, and biomass accumulation were evaluated under drastic (1.63 kPa for 6 min and 0.63 for 3 min) or moderate (1.32 kPa for 7 min and 0.86 kPa for 3 min) VPD fluctuation. The drastic VPD fluctuation induced a gradual decrease in stomatal conductance and thus CO 2 assimilation rate during the measurements, while moderate VPD fluctuation caused no reduction of these parameters. Thus, moderate VPD fluctuation maintained leaf expansion and the efficiency of CO 2 diffusion across the leaf surface, resulting in enhanced plant growth compared with drastic VPD fluctuation.
Organic Nutrient Supplementation Through Wastewater Treatment
Organic nutrients supplemented through wastewater treatment has great advantages in plant growth and yield and environmental sustainability. Researchers are applying a number of methods on the recovery of nutrients from wastewater and agricultural waste for organic greenhouse production (Voogt et al., 2011 ). Kechasov et al. have designed a closed hydroponic system with an integrated nitrification bioreactor. They compared plant development, fruit yield, and quality of tomatoes grown with the liquid by-product of biogas production from pig manure with greenhouse tomatoes grown with mineral fertilizers. The tomatoes grown with the organic waste-based fertilizer had a similar yield but poorer taste characteristics when compared with tomatoes grown with the high-mineral fertilizer. The plants grown with the organic waste-based treatment accumulated a higher amount of salts, especially tissue Cl − content. Fertilizers based on organic wastes change plant development toward a generative state and can partially recover the physiological and biochemical responses seen in plants grown under suboptimal fertilization conditions, suggesting that these fertilizers could be favored over mineral fertilizers with similar inorganic compositions. However, the use of organic waste-based fertilizers is less feasible than high-mineral fertilizers because of the lower quality of tomato fruits produced.
Chow et al. verified the viability of nickel electroplating industrial wastewater effluent diluted at different concentrations as a source of nutrient recycling using a hydroponic soilless cultivation system. The significant inhibition of the root and shoot elongation and reduction of photosynthetic pigments were accompanied by the profound morphological distortions in the xylem, phloem, and stomata. It was observed that beyond the maximum tolerable concentration level at 25% of wastewater effluent for hyacinth bean and 5% of wastewater effluent for pak choi. The accumulation of proline and upregulation of POD and APX activities were detected against the nickel electroplating industrial wastewater-induced oxidative stress injury in the plant models.
Celletti et al. investigated the possibility of using innovative fertilizer solutions in hydroponic systems for the growth of agricultural plants. Aqueous hydrothermal carbonized liquid (AHL) derived from cow manure digestate was chemically characterized for pH, electrical conductivity, mineral elements, and organic compounds. The AHL diluted with distilled water (1:30, 1:60, and 1:90, v/v) was used as a source of nutrients instead of standard hydroponic nutrient solution and bio-assayed using maize plants. The results indicated that the dilution ratio 1:30 of the AHL solution showed higher phytotoxicity while the increased dilutions (1:60 and 1:90) had a lower level of toxicity allowing plants to grow. It was clearly evident that higher dilution ratios contain insufficient essential nutrients for the plant, showing pronounced leaf chlorosis. Further studies recommended are identifying appropriate species-specific dilution ratios to supply both low levels of phytotoxins and adequate content of essential nutrients for appropriate plant growth and development.

Sustainable Crop Production Through Nutrient Recycling in Aquaponics
Cifuentes-Torres et al. mentioned that reclaimed water can, in theory, be used in aquaponics as it has been used as a water source in agriculture irrigation and aquaculture for many decades. They highlighted that there is an opportunity to use reclaimed water in aquaponics although there are still many questions that arise and more studies are needed to demonstrate that this technology is sustainable. There is the potential that toxic compounds such as certain toxic metals at low concentrations can function as food supplies in fish diets, under strict and controlled conditions. The presence of microalgae in aquaponic systems can be an advantage as it acts as both a food producer and wastewater treatment process. This Research Topic emphasized the studies with aquatic organisms and plants with the ability to metabolize contaminants without the risk to human health.
Lobanov et al. reported challenges of closed environment agriculture for resource-use optimization. The exploitation of readily available, soluble aquaculture effluent would be imperative for nutrient transfer in the hydroponic environment considering the role of microorganisms and the rhizosphere. In this regard, nutrient re-mineralization has to be adopted due to the challenges and carbon reduction and the additional costs associated with existing waste revalorization systems. They investigated micronutrient profiles of the re-mineralized effluent, traditional coupled aquaponics, and commercial hydroponic nutrient solutions were measured. Nutrient concentrations were significantly lower in the aquaculture-derived treatments than the commercial solution, while plant sap analysis did not follow the evidence of higher nutrient content in lettuce grown under excessive nutrient conditions. Lettuce grown in the commercial hydroponic nutrient solution likewise experienced deficiencies in Mg and Ca (young leaves) as well as Na and Si (both young and old leaves). The uptake of certain elements (Cu, Fe, Mg, S, and Zn) was greater across aquaponic treatments than initially predicted, however, Mn was universally absent from aquaponic treatments. B and P were especially low in the standard aquaponics treatment, i.e., fertilization with soluble RAS nutrients only. Together this suggests that the solids treatment system in parallel to RAS soluble effluent may be advantageous for aquaponic facilities seeking to maximize the benefits of the fish solids for plant nutrition. Nonetheless, iron remains the most capricious element to provide for plants. The evidence that neither the commercial solution nor aquaponic treatments were wholly successful in increasing iron uptake suggests a need for future studies to determine minimal “optimal” concentrations for plants and as well the real repercussions of deficiencies on crop yield and nutritional quality.
Implications and Future Challenges of Recycled Hydroponics
Recycled hydroponics has great implications in practice under controlled environment agriculture toward economic considerations and environmental sustainability. It is generally used in greenhouses and indoor farming plant factories for producing a range of high-value crops such as leafy and fruiting vegetables and medicinal plants under artificial light organized vertically. Nutrient recycling is important to achieve high resource use efficiency. Simple EC control of nutrient solution concentration would be sufficient for growing vegetables sustainably under recycled hydroponics (Bamsey et al., 2012 ; Jung et al., 2015 ; Chowdhury et al., 2021 ). As EC indicates the total ionic balance of solution, the specific mineral requirement of the plant is usually overlooked. In this regard, ion-selective electrodes (ISEs) have been used for improving plant growth and quality with efficient use of major nutrients (Rius-Ruiz et al., 2014 ; Cho et al., 2017 , 2018 ; Chowdhury et al., 2020 ). Therefore, the profitability of hydroponic farming can be achieved through yield maximization under recycled hydroponics based on either EC- or ISE- control of nutrient solution management.
The main concern of recycled hydroponics is the occurrence of pathogens due to the recirculating nature of the culture solution. Therefore, appropriate sterilization is essential for recycled hydroponics. Another important challenge of recycled hydroponics is the accumulation of inhibitory allelochemicals causing yield and quality reduction due to the autotoxicity phenomenon. Although a number of methods have been suggested to overcome the autotoxicity in several crops more appropriate recovery strategies are the future research need.
Author Contributions
MA, GN, and TA have made a substantial, direct, and intellectual contribution to the work, and approved the editorial for publication in Frontiers in Plant Science. MA prepared the original manuscript. GN and TA reviewed and edited revised manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- Asaduzzaman M., Talukder M. R., Tanaka H., Ueno M., Kawaguchi M., Yano S., et al. (2018). Production of low-potassium content melon through hydroponic nutrient management using perlite substrate. Front. Plant Sci. 9:1382. 10.3389/fpls.2018.01382 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Asao T., Kitazawa H., Ban T., Pramanik M. H. R. (2004). Search of autotoxic substances in some leaf vegetables. J. Japanese Soc. Hort. Sci. 73, 247–249. 10.2503/jjshs.73.247 [ DOI ] [ Google Scholar ]
- Asao T., Kitazawa H., Ushio K., Sueda Y., Ban T., Pramanik M. H. R. (2007). Autotoxicity in some ornamentals with means to overcome it. HortSci. 42, 1346–1350. 10.21273/HORTSCI.42.6.1346 [ DOI ] [ Google Scholar ]
- Bamsey M., Graham T., Thompson C., Berinstain A., Scott A., Dixon M. (2012). Ion-specific nutrient management in closed systems: the necessity for ion-selective sensors in terrestrial and space based agriculture and water management systems. Sensors 12, 13349–13392. 10.3390/s121013349 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cho W. J., Kim H. J., Jung D. H., Kang C. I., Choi G. L., Son J. E. (2017). An embedded system for automated hydroponic nutrient solution management. Trans. ASABE 60, 1083–1096. 10.13031/trans.12163 [ DOI ] [ Google Scholar ]
- Cho W. J., Kim H. J., Jung D. H., Kim D. W., Ahn T. I., Son J. E. (2018). On-site ion monitoring system for precision hydroponic nutrient management. Comput. Electron. Agric. 146, 51–58. 10.1016/j.compag.2018.01.019 [ DOI ] [ Google Scholar ]
- Chowdhury M., Islam M. N., Reza M. N., Ali M., Rasool K., Kiraga S., et al. (2021). Sensor-based nutrient recirculation for aeroponic lettuce cultivation. J. Biosyst. Eng. 46, 81–92. 10.1007/s42853-021-00089-8 [ DOI ] [ Google Scholar ]
- Chowdhury M., Jang B. E., Kabir M. S. N., Kim Y. J., Na K. D., Park S. B., et al. (2020). Factors affecting the accuracy and precision of ion-selective electrodes for hydroponic nutrient supply systems. Acta Hortic. 1296, 997–1004. 10.17660/ActaHortic.2020.1296.126 [ DOI ] [ Google Scholar ]
- Ciriello M., Formisano L., Pannico A., El-Nakhel C., Fascella G., Duri L. G., et al. (2021). Nutrient solution deprivation as a tool to improve hydroponics sustainability: yield, physiological, and qualitative response of lettuce. Agron 11,1469. 10.3390/agronomy11081469 [ DOI ] [ Google Scholar ]
- Gruda N. (2009). Do soil-less culture systems have an influence on product quality of vegetables? J. Appl. Bot. Food Qual. 82, 141–147. 10.18452/9433 [ DOI ] [ Google Scholar ]
- Jensen M. H. (1997). Hydroponics. HortSci. 32, 1018–1021. [ Google Scholar ]
- Jung D. H., Kim H. J., Choi G. L., Ahn T. I., Son J. E., Sudduth K. A. (2015). Automated lettuce nutrient solution management using an array of ion-selective electrodes. Trans. ASABE 58, 1309–1319. 10.13031/trans.58.11228 [ DOI ] [ Google Scholar ]
- Kitazawa H., Asao T., Ban T., Pramanik M. H. R., Hosoki T. (2005). Autotoxicity of root exudates from strawberry in hydroponic culture. J. Hortic. Sci. Biotech. 80, 677–680. 10.2503/hortj.UTD-R009 [ DOI ] [ Google Scholar ]
- Lee J. G., Lee B. Y., Lee H. J. (2006). Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce ( Lactuca sativa L.). Sci. Hortic. 110, 119–128. 10.1016/j.scienta.2006.06.013 [ DOI ] [ Google Scholar ]
- Nederhoff E., Stanghellini C. (2010). Water use efficiency of tomatoes. Pract. Hydrop. Greenhouses 115,52. Available online at: https://edepot.wur.nl/156932 [ Google Scholar ]
- Resh H. M. (2012). Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower. Boca Raton, FL: CRC Press. [ Google Scholar ]
- Rius-Ruiz F. X., Andrade F. J., Riu J., Rius F. X. (2014). Computer-operated analytical platform for the determination of nutrients in hydroponic systems. Food Chem. 147, 92–97. 10.1016/j.foodchem.2013.09.114 [ DOI ] [ PubMed ] [ Google Scholar ]
- Savvas D., Gianquinto G., Tuzel Y., Gruda N. (2013). “Soilless culture,” in Good Agricultural Practices Principles for Greenhouse Vegetable Production in the Mediterranean Region (FAO Paper: ), 303–354. [ Google Scholar ]
- Son J. E., Kim H. J., Ahn T. I. (2020). “Hydroponic systems,” in: Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd Edn , eds T. Kozai, G. Niu, and M. Takagaki (Cambridge, MA: Academic Press; ), 273–283. 10.1016/B978-0-12-816691-8.00020-0 [ DOI ] [ Google Scholar ]
- Talukder M. R., Asaduzzaman M., Tanaka H., Asao T. (2018). Light-emitting diodes and exogenous amino acids application improve growth and yield of strawberry plants cultivated in recycled hydroponics. Sci. Hortic. 239, 93–103. 10.1016/j.scienta.2018.05.033 [ DOI ] [ Google Scholar ]
- Voogt W., de Visser P. H. E., van Winkel A., Cuijpers W. J. M., van de Burgt G. J. H. M. (2011). Nutrient management in organic greenhouse production: navigation between constraints. Acta Hortic. 915, 75–82. 10.17660/ActaHortic.2011.915.9 [ DOI ] [ Google Scholar ]
- Yu J. Q., Matsui Y. (1993). Extraction and identification of phytotoxic substances accumulated in nutrient solution for the hydroponic culture of tomato. Soil Sci. Plant Nutr. 39, 691–700. 10.1080/00380768.1993.10419186 [ DOI ] [ Google Scholar ]
- Yu J. Q., Matsui Y. (1994). Phytotoxic substances in root exudates of cucumber ( Cucumis sativus L.). J. Chem. Ecol. 20, 21–31. 10.1007/bf02065988 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (159.9 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Advertisement
Recent advances and perspectives in the treatment of hydroponic wastewater: a review
- Review paper
- Published: 21 October 2020
- Volume 19 , pages 945–966, ( 2020 )
Cite this article

- Amina Richa ORCID: orcid.org/0000-0002-1353-4517 1 ,
- Sami Touil ORCID: orcid.org/0000-0002-8226-4194 1 ,
- Meriem Fizir ORCID: orcid.org/0000-0003-0118-7017 2 &
- Vicente Martinez 3
5260 Accesses
61 Citations
11 Altmetric
Explore all metrics
Rapid development of hydroponic farming which is soilless cultivation method of growing plant using mineral nutrient solution dissolved in water produces large amount of wastewater rich in nutrients and organic matters thus imposes great harms to human and environment, if the waste nutrient solution is not correctly treated. The objective of this review is to present information concerning hydroponic systems, including: the different classes and methods of operation; advantages and drawbacks and the recent approaches and development in hydroponic wastewater treatments. Particular emphasis has been placed on removal of root exudates from reused waste nutrient solution in closed system. The reviewed technologies for nutrient removal or recovery include denitrification, microalgae cultivation, constructed wetlands and activated carbon methods. The alternatives byproducts i.e. biogas as agriculture fertilizer for hydroponic cultivation to attain sustainable agriculture was further highlighted. In addition, current challenges and future prospects in this field are carried out. About 118 published studies are reviewed in this paper. It is evident from the literature survey articles that activated carbon is the most frequently studied for the nutrient recovery of hydroponic wastewater.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Modified from ref (Seo et al. 2008 )

Similar content being viewed by others

Review of current hydroponic food production practices and the potential role of bioelectrochemical systems

Constructed Wetlands: An Emerging Green Technology for Phosphorus Treatment in Industrial Wastewater

Emerging Technologies for Separation and Recycle of Phosphorous from Sewage Sludge for Hydroponic Farming System
Alipio MI, Cruz AEMD, Doria JDA, Fruto RMS (2019) On the design of nutrient film technique hydroponics farm for smart agriculture. Eng Agric Environ Food 12:315–324
Google Scholar
Al-Maskri A, Al-Kharusi L, Al-Miqbali H, Khan MM (2010) Effects of salinity stress on growth of lettuce (Lactuca sativa) under closed-recycle nutrient film technique. Int J Agric Biol 12:377–380
CAS Google Scholar
Asaduzzaman M, Asao T (2012) Autotoxicity in beans and their allelochemicals. Sci Hortic 134:26–31
Asaduzzaman M, Asao T (2020) Autotoxicity in strawberry under recycled hydroponics and its mitigation methods. Hortic J 89:124–137
Asaduzzaman M, Kobayashi Y, Mondal MF, Ban T, Matsubara H, Adachi F, Asao T (2013) Growing carrots hydroponically using perlite substrates. Sci Hortic 159:113–121
Asao T, Hasegawa K, Sueda Y, Tomita K, Taniguchi K, Hosoki T, Pramanik M, Matsui Y (2003) Autotoxicity of root exudates from taro. Sci Hortic 97:389–396
Awad YM, Lee S-E, Ahmed MBM, Vu NT, Farooq M, Kim IS, Kim HS, Vithanage M, Usman ARA, Al-Wabel M (2017) Biochar, a potential hydroponic growth substrate, enhances the nutritional status and growth of leafy vegetables. J Clean Prod 156:581–588
Breś W (2009) Estimation of nutrient losses from open fertigation systems to soil during horticultural plant cultivation. Pol J Environ Stud 18:341–345
Carmassi G, Incrocci L, Malorgio M, Tognoni F, Pardossi A (2002) A simple model for salt accumulation in closed-loop hydroponics. In: VI International symposium on protected cultivation in mild winter climate: product and process innovation, vol 614, pp 149–154
Carmassi G, Incrocci L, Maggini R, Malorgio F, Tognoni F, Pardossi A (2005) Modeling salinity build-up in recirculating nutrient solution culture. J Plant Nutr 28:431–445
Castellar J, Formosa J, Fernández AI, Jové P, Bosch MG, Morató J, Brix H, Arias CA (2019) Cork as a sustainable carbon source for nature-based solutions treating hydroponic wastewaters–preliminary batch studies. Sci Total Environ 650:267–276
Charoenpakdee S (2014) Using animal manure to grow lettuce ( Lactuca sativa L.) in a homemade hydroponics system. Asia-Pacific J Sci Technol 19:256–261
Choi B, Lee SS, Awad YM, Ok YS (2011) Feasibility of reclaimed wastewater and waste nutrient solution for crop production in Korea. Korean J Environ Agric 30:118–124
Choi E-Y, Yoon Y-H, Choi K-Y, Lee Y-B (2015) Environmentally sustainable production of tomato in a coir substrate hydroponic system using a frequency domain reflectometry sensor. Hortic Environ Biotechnol 56:167–177
Chow K, Price T, Hanger B (1992) Nutritional requirements for growth and yield of strawberry in deep flow hydroponic systems. Sci Hortic 52:95–104
Chow K, Price T, Hanger B (2002) Effects of nitrogen, potassium, calcium concentrations and solution temperatures on the growth and yield of strawberry cv. Redgauntlet in a nutrient film (NFT) hydroponic system. In: XXVI international horticultural congress: protected cultivation 2002: in search of structures, systems and plant materials for 633, pp 315–327
Chu W, Gao N, Yin D, Krasner SW, Mitch WA (2014) Impact of UV/H2O2 pre-oxidation on the formation of haloacetamides and other nitrogenous disinfection byproducts during chlorination. Environ Sci Technol 48:12190–12198
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19:292–305
Daud M, Handika V, Bintoro A (2018) Design and realization of fuzzy logic control for ebb and flow hydroponic system. Int J Sci Technol Res 7:138–144
Dorais M, Dubé Y (2009) Managing greenhouse organic wastes: a holistic approach. In: International symposium on high technology for greenhouse systems: GreenSys2009, vol 893, pp 183–197
Du Z, Jia R, Li C, Cui P, Song W, Liu J (2020) Pilot-scale UV/H2O2-BAC process for drinking water treatment—analysis and comparison of different activated carbon columns. Chem Eng J 382:123044
Farrar J, Hawes M, Jones D, Lindow S (2003) How roots control the flux of carbon to the rhizosphere. Ecology 84:827–837
Gagnon V, Maltais-Landry G, Puigagut J, Chazarenc F, Brisson J (2010) Treatment of hydroponics wastewater using constructed wetlands in winter conditions. Water Air Soil Pollut 212:483–490
Gao F, Zhang H, Yang F, Qiang H, Li H, Zhang R (2013) Study of an innovative anaerobic (A)/oxic (O)/anaerobic (A) bioreactor based on denitrification–anammox technology treating low C/N municipal sewage. Chem Eng J 232:65–73
Gentry M (2019) Local heat, local food: integrating vertical hydroponic farming with district heating in Sweden. Energy 174:191–197
Goddek S, Joyce A, Kotzen B, Burnell GM (2019) Aquaponics food production systems. Springer, Berlin
Grasselly D, Merlin G, Sédilot C, Vanel F, Dufour G, Rosso L (2004) Denitrification of soilless tomato crops run-off water by horizontal subsurface constructed wetlands. In: International conference on sustainable greenhouse systems-Greensys2004, vol 691, pp 329–332
Grewal HS, Maheshwari B, Parks SE (2011) Water and nutrient use efficiency of a low-cost hydroponic greenhouse for a cucumber crop: an Australian case study. Agric Water Manag 98:841–846
Guilayn F, Benbrahim M, Rouez M, Crest M, Patureau D, Jimenez J (2020) Humic-like substances extracted from different digestates: first trials of lettuce biostimulation in hydroponic culture. Waste Manag 104:239–245
Hosseinzadeh S, Verheust Y, Bonarrigo G, Van Hulle S (2017a) Closed hydroponic systems: operational parameters, root exudates occurrence and related water treatment. Rev Environ Sci Bio/Technol 16:59–79
Hosseinzadeh S, Bonarrigo G, Verheust Y, Roccaro P, Van Hulle S (2017b) Water reuse in closed hydroponic systems: comparison of GAC adsorption, ion exchange and ozonation processes to treat recycled nutrient solution. Aquacult Eng 78:190–195
Hosseinzadeh S, Testai D, BKheet M, De Graeve J, Roccaro P, Van Hulle S (2019a) Degradation of root exudates in closed hydroponic systems using UV/H2O2: kinetic investigation, reaction pathways and cost analysis. Sci Total Environ 687:479–487
Hosseinzadeh S, Liu Z, De Graeve J, BKheet M, Libbrecht W, De Clercq J, Van Hulle S (2019b) Recirculating water treatment in closed hydroponic systems: assessment of granular activated carbon and soft templated mesoporous carbon for adsorptive removal of root exudates. Environmental Processes 6:1–23
Hultberg M, Carlsson AS, Gustafsson S (2013) Treatment of drainage solution from hydroponic greenhouse production with microalgae. Biores Technol 136:401–406
Huo S, Liu J, Addy M, Chen P, Necas D, Cheng P, Li K, Chai H, Liu Y, Ruan R (2020) The influence of microalgae on vegetable production and nutrient removal in greenhouse hydroponics. J Clean Prod 243:118563
Ikeura H, Tsukada K, Tamaki M (2017) Effect of microbubbles in deep flow hydroponic culture on Spinach growth. J Plant Nutr 40:2358–2364
Jin P, Jin X, Wang X, Feng Y, Wang XC (2013) Biological activated carbon treatment process for advanced water and wastewater treatment. Biomass Now-Cultiv Utiliz 153–192
Jones JB Jr (1982) Hydroponics: its history and use in plant nutrition studies. J Plant Nutr 5:1003–1030
Karakaş C, Özçimen D, İnan B (2017) Potential use of olive stone biochar as a hydroponic growing medium. J Anal Appl Pyrol 125:17–23
Kitaya Y, Hirai H, Wei X, Islam A, Yamamoto M (2008) Growth of sweetpotato cultured in the newly designed hydroponic system for space farming. Adv Space Res 41:730–735
Kitazawa H, Asao T, Ban T, Pramanik M, Hosoki T (2005) Autotoxicity of root exudates from strawberry in hydroponic culture. J Hortic Sci Biotechnol 80:677–680
Koide S, Satta N (2004) Separation performance of ion-exchange membranes for electrolytes in drainage nutrient solutions subjected to electrodialysis. Biosys Eng 87:89–97
Kozai T (2018) Smart plant factory: the next generation indoor vertical farms. Springer, Berlin
Krishnasamy K, Nair J, Bäuml B (2012) Hydroponic system for the treatment of anaerobic liquid. Water Sci Technol 65:1164–1171
Kumar RR, Cho JY (2014) Reuse of hydroponic waste solution. Environ Sci Pollut Res 21:9569–9577
Lee S, Lee J (2015) Beneficial bacteria and fungi in hydroponic systems: types and characteristics of hydroponic food production methods. Sci Hortic 195:206–215
Lee JG, Lee BY, Lee HJ (2006) Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce ( Lactuca sativa L.). Sci Hortic 110:119–128
Lee YH, Yoon CS, Park NI, Yeoung YR (2015) Influence of various nutrient concentrations on the growth and yield of summer strawberry cultivars cultivated in a hydroponic system. Hortic Environ Biotechnol 56:421–426
Lee JY, Rahman A, Behrens J, Brennan C, Ham B, Kim HS, Nho CW, Yun S-T, Azam H, Kwon MJ (2018) Nutrient removal from hydroponic wastewater by a microbial consortium and a culture of Paracercomonas saepenatans . New Biotechnol 41:15–24
Lee C, Kim DS, Kwack Y, Chun C (2020) Waste nutrient solution as an alternative fertilizer in curled mallow cultivation. J Agric Sci 12:55–66
Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W, Huo S, Cheng P, Liu J, Addy M (2019) Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour Technol 291:121934
Lind OP, Hultberg M, Bergstrand K-J, Larsson-Jönsson H, Caspersen S, Asp H (2020) Biogas digestate in vegetable hydroponic production: pH dynamics and pH management by controlled nitrification. Waste Biomass Valorization 1–11
Liu H, Jiang W, Wan D, Qu J (2009) Study of a combined heterotrophic and sulfur autotrophic denitrification technology for removal of nitrate in water. J Hazard Mater 169:23–28
Liu Z, Hosseinzadeh S, Wardenier N, Verheust Y, Chys M, Hulle SV (2019) Combining ozone with UV and H2O2 for the degradation of micropollutants from different origins: lab-scale analysis and optimization. Environ Technol 40:3773–3782
Lopez-Galvez F, Allende A, Pedrero-Salcedo F, Alarcon JJ, Gil MI (2014) Safety assessment of greenhouse hydroponic tomatoes irrigated with reclaimed and surface water. Int J Food Microbiol 191:97–102
Magwaza ST, Magwaza LS, Odindo AO, Mditshwa A (2020a) Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: a review. Sci Total Environ 698:134154
Magwaza ST, Magwaza LS, Odindo AO, Mditshwa A, Buckley C (2020b) Evaluating the feasibility of human excreta-derived material for the production of hydroponically grown tomato plants—part II: growth and yield. Agric Water Manag 234:106115
Magwaza ST, Magwaza LS, Odindo AO, Mashilo J, Mditshwa A, Buckley C (2020c) Evaluating the feasibility of human excreta-derived material for the production of hydroponically grown tomato plants-part I: photosynthetic efficiency, leaf gas exchange and tissue mineral content. Agric Water Manag 234:106114
Mahjoor F, Ghaemi AA, Golabi MH (2016) Interaction effects of water salinity and hydroponic growth medium on eggplant yield, water-use efficiency, and evapotranspiration. Int Soil Water Conserv Res 4:99–107
Manos D-P, Xydis G (2019) Hydroponics: are we moving towards that direction only because of the environment? A discussion on forecasting and a systems review. Environ Sci Pollut Res 26:12662–12672
Matsui S, Yamamoto R (1986) A new method of sulphur denitrification for sewage treatment by a fluidized bed reactor. Water Sci Technol 18:355–362
Maucieri C, Nicoletto C, Van Os E, Anseeuw D, Van Havermaet R, Junge R (2019) Hydroponic technologies. In: Goddek S, Joyce A, Kotzen B, Burnell G (eds) Aquaponics food production systems. Springer, Berlin, pp 77–110
Mielcarek A, Rodziewicz J, Janczukowicz W, Dobrowolski A (2019) Analysis of wastewater generated in greenhouse soilless tomato cultivation in central Europe. Water 11:2538
Mikkelson KM, Homme CL, Li D, Sharp JO (2015) Propane biostimulation in biologically activated carbon (BAC) selects for bacterial clades adept at degrading persistent water pollutants. Environ Sci Process Impacts 17:1405–1414
Miyama Y, Kawashima Y, Ogawa J, Uekusa H, Okamoto T, Kita N, Sunada K, Hashimoto K (2013) Inactivation of bacterial wilt in closed soilless cultivation by photocatalytic treatment and silver. Environ Control Biol 51:173–178
Mohammed S (2018) Tomorrow’s agriculture: “NFT Hydroponics”—grow within your budget. Springer, Berlin
Molders K, Quinet M, Decat J, Secco B, Dulière E, Pieters S, van der Kooij T, Lutts S, Van Der Straeten D (2012) Selection and hydroponic growth of potato cultivars for bioregenerative life support systems. Adv Space Res 50:156–165
Mosa A, El-Banna MF, Gao B (2016) Biochar filters reduced the toxic effects of nickel on tomato ( Lycopersicon esculentum L.) grown in nutrient film technique hydroponic system. Chemosphere 149:254–262
Mowa E, Akundabweni L, Chimwamurombe P, Oku E, Mupambwa HA (2017) The influence of organic manure formulated from goat manure on growth and yield of tomato ( Lycopersicum esculentum ). Afr J Agric Res 12:3061–3067
Mupambwa HA, Namwoonde AS, Liswaniso GM, Hausiku MK, Ravindran B (2019) Biogas digestates are not an effective nutrient solution for hydroponic tomato ( Lycopersicon esculentum L.) production under a deep water culture system. Heliyon 5:e02736
Muro J, Diaz V, Goni J, Lamsfus C (1997) Comparison of hydroponic culture and culture in a peat/sand mixture and the influence of nutrient solution and plant density on seed potato yields. Potato Res 40:431–438
Mwakabole EC, Rwiza MJ, Njau KN (2020) Column design for groundwater hardness removal using cashew nut shells activated carbon with potential application in low-income communities
Nandwani D (2018) Urban horticulture. Springer, Berlin
Neocleous D, Savvas D (2016) NaCl accumulation and macronutrient uptake by a melon crop in a closed hydroponic system in relation to water uptake. Agric Water Manag 165:22–32
Neocleous D, Nikolaou G, Ntatsi G, Savvas D (2020) Impact of chelated or inorganic manganese and zinc applications in closed hydroponic bean crops on growth, yield, photosynthesis, and nutrient uptake. Agronomy 10:881
Oller I, Malato S, Sánchez-Pérez J (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409:4141–4166
Page V, Feller U (2013) Selection and hydroponic growth of bread wheat cultivars for bioregenerative life support systems. Adv Space Res 52:536–546
Park C-J, Kim K-H, Yoo K-Y, Ok Y-S, Yang J-E (2005) Recycling of hydroponic waste solution for red pepper ( Capsicum annum L.) growth. Korean J Environ Agric 24:24–28
Park J, Craggs R, Sukias J (2008a) Treatment of hydroponic wastewater by denitrification filters using plant prunings as the organic carbon source. Biores Technol 99:2711–2716
Park W-Y, Seo D-C, Lim J-S, Park S-K, Cho J-S, Heo J-S, Yoon H-S (2008b) Optimum configuration, filter media depth and wastewater load of small-scale constructed wetlands for treating the hydroponic waste solution in greenhouses. Korean J Environ Agric 27:217–224
Park J, Craggs R, Sukias J (2009) Removal of nitrate and phosphorus from hydroponic wastewater using a hybrid denitrification filter (HDF). Biores Technol 100:3175–3179
Park J-H, Kim S-H, Delaune RD, Cho J-S, Heo J-S, Ok YS, Seo D-C (2015) Enhancement of nitrate removal in constructed wetlands utilizing a combined autotrophic and heterotrophic denitrification technology for treating hydroponic wastewater containing high nitrate and low organic carbon concentrations. Agric Water Manag 162:1–14
Patel P, Muteen A, Mondal P (2019) Treatment of greywater using waste biomass derived activated carbons and integrated sand column. Sci Total Environ 711:134586
Peng M, Li H, Kang X, Du E, Li D (2017) Photo-degradation ibuprofen by UV/H2O2 process: response surface analysis and degradation mechanism. Water Sci Technol 75:2935–2951
Power SD, Jones CL (2016) Anaerobically digested brewery effluent as a medium for hydroponic crop production—the influence of algal ponds and pH. J Clean Prod 139:167–174
Pramanik M, Nagai M, Asao T, Matsui Y (2000) Effects of temperature and photoperiod on phytotoxic root exudates of cucumber ( Cucumis sativus ) in hydroponic culture. J Chem Ecol 26:1953–1967
Prystay W, Lo K (2001) Treatment of greenhouse wastewater using constructed wetlands. J Environ Sci Health, Part B 36:341–353
Qadeer A, Butt SJ, Asam HM, Mehmood T, Nawaz MK, Haidree SR (2020) 3. Hydroponics as an innovative technique for lettuce production in greenhouse environment. Pure Appl Biol (PAB) 9:20–26
Rijsberman FR (2006) Water scarcity: Fact or fiction? Agric Water Manag 80:5–22
Rodziewicz J, Mielcarek A, Janczukowicz W, Jóźwiak T, Struk-Sokołowska J, Bryszewski K (2019) The share of electrochemical reduction, hydrogenotrophic and heterotrophic denitrification in nitrogen removal in rotating electrobiological contactor (REBC) treating wastewater from soilless cultivation systems. Sci Total Environ 683:21–28
Ronga D, Setti L, Salvarani C, De Leo R, Bedin E, Pulvirenti A, Milc J, Pecchioni N, Francia E (2019) Effects of solid and liquid digestate for hydroponic baby leaf lettuce ( Lactuca sativa L.) cultivation. Sci Hortic 244:172–181
Rufí-Salís M, Petit-Boix A, Villalba G, Sanjuan-Delmás D, Parada F, Ercilla-Montserrat M, Arcas-Pilz V, Muñoz-Liesa J, Rieradevall J, Gabarrell X (2020a) Recirculating water and nutrients in urban agriculture: an opportunity towards environmental sustainability and water use efficiency? J Clean Prod 261:121213
Rufí-Salís M, Calvo MJ, Petit-Boix A, Villalba G, Gabarrell X (2020b) Exploring nutrient recovery from hydroponics in urban agriculture: an environmental assessment. Resour Conserv Recycl 155:104683
Saaid M, Yahya N, Noor M, Ali MM (2013) A development of an automatic microcontroller system for Deep Water Culture (DWC). In: 2013 IEEE 9th international colloquium on signal processing and its applications. IEEE, pp 328–332
Salgot M, Folch M, Huertas E, Tapias J, Avellaneda D, Girós G, Brissaud F, Vergés C, Molina J, Pigem J (2002) Comparison of different advanced disinfection systems for wastewater reclamation. Water Sci Technol Water Supply 2:213–218
Santos FM, Pires JC (2018) Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Biores Technol 267:725–731
Sardare MD, Admane SV (2013) A review on plant without soil-hydroponics. Int J Res Eng Technol 2:299–304
Savvas D, Pappa V, Kotsiras A, Gizas G (2005) NaCl accumulation in a cucumber crop grown in a completely closed hydroponic system as influenced by NaCl concentration in irrigation water. Eur J Hortic Sci 70:217
Savvas D, Mantzos N, Barouchas P, Tsirogiannis I, Olympios C, Passam H (2007a) Modelling salt accumulation by a bean crop grown in a closed hydroponic system in relation to water uptake. Sci Hortic 111:311–318
Savvas D, Stamati E, Tsirogiannis I, Mantzos N, Barouchas P, Katsoulas N, Kittas C (2007b) Interactions between salinity and irrigation frequency in greenhouse pepper grown in closed-cycle hydroponic systems. Agric Water Manag 91:102–111
Saxena P, Bassi A (2013) Removal of nutrients from hydroponic greenhouse effluent by alkali precipitation and algae cultivation method. J Chem Technol Biotechnol 88:858–863
Sengupta A, Banerjee H (2012) Soil-less culture in modern agriculture. World J Sci Technol 2:103–108
Seo DC, Hwang SH, Kim HJ, Cho JS, Lee HJ, DeLaune RD, Jugsujinda A, Lee ST, Seo JY, Heo JS (2008) Evaluation of 2-and 3-stage combinations of vertical and horizontal flow constructed wetlands for treating greenhouse wastewater. Ecol Eng 32:121–132
Seo D-C, Park J-H, Cheon Y-S, Park S-K, Kim A-R, Lee W-G, Lee S-W, Lee S-T, Cho J-S, Heo J-S (2010) Treatment efficiency of pollutants in constructed wetlands under different hydroponic wastewater injection methods and characteristic of filter media. Korean J Environ Agric 29:146–151
Sharma N, Acharya S, Kumar K, Singh N, Chaurasia O (2018) Hydroponics as an advanced technique for vegetable production: an overview. J Soil Water Conserv 17:364–371
Souza SV, Gimenes RMT, Binotto E (2019) Economic viability for deploying hydroponic system in emerging countries: a differentiated risk adjustment proposal. Land Use Policy 83:357–369
Surendran U, Chandran C, Joseph E (2017) Hydroponic cultivation of Mentha spicata and comparison of biochemical and antioxidant activities with soil-grown plants. Acta Physiol Plant 39:26
Takemura K, Endo R, Shibuya T, Kitaya Y (2020) Application of biogas digestate as a nutrient solution for the hydroponic culture of chrysanthemum morifolium ramat with rockwool substrate. Waste Biomass Valor 11:2645–2650
Tan XW, Ikeda H, Oda M (2000) The absorption, translocation, and assimilation of urea, nitrate or ammonium in tomato plants at different plant growth stages in hydroponic culture. Sci Hortic 84:275–283
Van Rijn J, Tal Y, Schreier HJ (2006) Denitrification in recirculating systems: theory and applications. Aquacult Eng 34:364–376
Vrhovšek D, Kukanja V, Bulc T (1996) Constructed wetland (CW) for industrial waste water treatment. Water Res 30:2287–2292
Wang L, Guo S, Wang Y, Yi D, Wang J (2019) Poultry biogas slurry can partially substitute for mineral fertilizers in hydroponic lettuce production. Environ Sci Pollut Res 26:659–671
Xydis GA, Liaros S, Botsis K (2017) Energy demand analysis via small scale hydroponic systems in suburban areas—an integrated energy-food nexus solution. Sci Total Environ 593:610–617
Yamamoto-Ikemoto R, Komori T, Nomuri M, Ide Y, Matsukami T (2000) Nitrogen removal from hydroponic culture wastewater by autotrophic denitrification using thiosulfate. Water Sci Technol 42:369–376
Yang L, Giannis A, Chang VW-C, Liu B, Zhang J, Wang J-Y (2015) Application of hydroponic systems for the treatment of source-separated human urine. Ecol Eng 81:182–191
Yu JQ, Lee KS, Matsui Y (1993) Effect of the addition of activated charcoal to the nutrient solution on the growth of tomato in hydroponic culture. Soil Sci Plant Nutr 39:13–22
Zhang J, Wang X, Zhou Q (2017) Co-cultivation of Chlorella spp and tomato in a hydroponic system. Biomass Bioenergy 97:132–138
Zubair M, Wang S, Zhang P, Ye J, Liang J, Nabi M, Zhou Z, Tao X, Chen N, Sun K (2020) Biological nutrient removal and recovery from solid and liquid livestock manure: recent advance and perspective. Biores Technol 301:122823
Download references
Acknowledgements
This paper is supported by the PRIMA programme under Grant Agreement No. 1821, WATERMED 4.0, Call 2018 Section 1 Water. The PRIMA programme is supported by the European Union. We appreciate the support provided by General Directorate for Scientific Research and Technological Development (DGRSDT).
Author information
Authors and affiliations.
Research Laboratory of Agricultural Production and Sustainable Development of Natural Resources, University of Djilali Bounaama, Khemis Miliana, Algeria
Amina Richa & Sami Touil
Laboratoire de Valorisation des Substances Naturelles, Université Djilali Bounaâma, Khemis-Miliana, Algeria
Meriem Fizir
Department of Plant Nutrition, CEBAS-CSIC, Murcia, Spain
Vicente Martinez
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Amina Richa , Sami Touil or Meriem Fizir .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Richa, A., Touil, S., Fizir, M. et al. Recent advances and perspectives in the treatment of hydroponic wastewater: a review. Rev Environ Sci Biotechnol 19 , 945–966 (2020). https://doi.org/10.1007/s11157-020-09555-9
Download citation
Received : 11 August 2020
Accepted : 10 October 2020
Published : 21 October 2020
Issue Date : December 2020
DOI : https://doi.org/10.1007/s11157-020-09555-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hydroponic system
- Hydroponic wastewaters
- Organic fertilizers
- Nutrient removal and recovery
- Root exudates
- Find a journal
- Publish with us
- Track your research

COMMENTS
Hydroponic, is an alternative agriculture production system that focuses on climate resilience, efficient resource utilization, and disease-free crop production [1]. In hydroponics, instead of soil, plants receive a nutrient-rich water solution directly, providing them with the essential elements they need for growth.
Modern hydroponic systems have evolved substantially since the early 20th century, with advancements in system design, automation, nutrient management, and environmental control enhancing ...
This work was supported by the Knowledge Economy Skills Scholarships (KESS 2) led by Bangor University on behalf of the HE sector in Wales, and partly funded by the Welsh Government's European Social Fund (ESF) convergence programme for West Wales and the Valleys and the UK Biotechnology and Biological Sciences Research Council (grant number ...
According to the Food and Agriculture Organization of the United Nations, the world population will reach nine billion people in 2050, of which 75% will live in urban settlements. One of the biggest challenges will be meeting the demand for food, as farmland is being lost to climate change, water scarcity, soil pollution, among other factors. In this context, hydroponics, an agricultural ...
This work conceptualizes the integration of bioelectrochemical systems and hydroponics with a view to improving the efficiency and sustainability of hydroponics.
Hydroponic research on lettuce, spinach and other leafy. vegetables. ... In this work, guppy fishes were used in aquaponics as a source of nutrients. For hydroponics, the N: P: K fertilizer named ...
The Fe supply (in terms of quantities and usable forms) is an issue also for soilless crops. Several pieces of research have compared in hydroponic solutions the efficiency of different organic ligands complexed with Fe as micronutrient source for plants (Pinton et al., 1999; Tomasi et al., 2009, 2013, 2014).
Nutrient Recycling in Hydroponics. Hydroponics is a method of growing plants in soilless media or nutrient solution containing all essential mineral nutrients that potentially lead to yield and quality improvement (Gruda, 2009).In principle, nutrient solutions used in hydroponics can either be reused or discarded (Jensen, 1997; Nederhoff and Stanghellini, 2010).
Chapters explore the drawbacks and advantages of using hydroponics as a soilless crop production system and provide a wider view of science, technology, and economic impact of hydroponics. This book is a valuable resource to agriculturists, horticulturalists, greenhouse and nursery managers, and professionals involved with the production of plants.
Hydroponics is method of farming where crops can be grown in nutrient fortified water and without the use of soil (soilless culture) (Maucieri et al. 2019).This technology has been in existence since the eighteen century (Mohammed 2018) and commercial scale hydroponic systems were developed in the 1940s (Lee and Lee 2015).The Greek words, hydro and ponos meaning water and working respectively ...